-
上颈椎畸形伴寰枢关节脱位或不稳常导致脊髓压迫,严重影响病人的生活质量。目前脊柱外科治疗这种颅颈交界区疾病的标准术式是后路枕颈融合术,但传统枕颈融合的内植物存在置钉困难、缺乏良好解剖形态匹配、无孔隙结构等缺陷,无法直接骨长入,只能进行植骨融合,存在融合失败的风险[1]。有学者发现多孔结构对骨长入有积极的促进作用,并利用3D打印技术设计制造多孔植入物来解决骨长入问题[2-3]。我院脊柱外科团队利用3D打印等数字医学技术,研制出一款集固定、置钉、融合为一体的3D打印个体化枕颈融合器(专利号:ZL201721220260.5),并将其骨接触面设计为仿骨小梁的多孔结构。但这种多孔结构与颅骨、颈椎后弓表面皮质骨能否像3D打印多孔髋臼杯一样获得良好骨长入,尚未见相关报道。为此,我们利用兔长骨皮质骨模拟颅骨与颈椎后弓皮质骨进行实验,旨在探讨3D打印多孔钛板能否与处理后的皮质骨表面形成有效骨长入从而获得融合,并确定适宜骨长入的孔隙率,为3D打印枕颈融合器在骨性融合方面提供理论支持。现作报道。
HTML
-
健康新西兰大白兔18只,兔龄6~8个月,雌雄不限,体质量3.0~3.5 kg,购于南京市江宁区青龙山动物繁殖场[生产许可证号:SCXK(苏)2017-0011;实验动物合格证号:No 201803587],分笼饲养。3D打印多孔钛板(北京爱康公司提供打印),3D打印机(上海复志公司),硬组织切片机(德国艾卡特公司),硬组织磨片机(德国EXAKT公司),螺旋CT、X光机(德国西门子公司)。10%水合氯醛,VG染液(上海己博公司)。
-
使用64排螺旋CT对实验兔进行0.5 mm层厚连续薄层扫描,将扫描的股骨干数据以DICOM格式文件导出,并使用三维重建软件Mimics 17.0对数据进行重建。将股骨干3D模型数据传导入ideaMaker软件进行切片,切片完成后传入3D打印机进行模型打印。
-
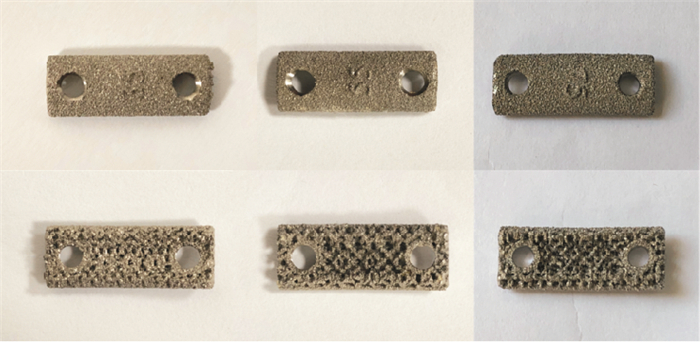
测量股骨干3D模型的直径、长度等参数。根据兔股骨干形态和所测参数设计多孔钛板形状及尺寸:主体形状为长方形,长10 mm,宽5 mm,厚2 mm,螺孔Φ2.4 mm。钛板主体结构分为两层,即骨接触面多孔层(厚度1.4 mm)和非骨接触面层(厚度0.6 mm),孔径600 μm,孔隙率分为35%、55%和75%。最后利用电子束融化法进行打印(见图 1、2)。
-
实验兔随机分为A、B、C组,各6只,分别植入35%、55%、75%孔隙率的3D打印多孔钛板。术前备皮,禁食、水12 h,术前30 min青霉素肌肉注射预防感染。10%水合氯醛溶液(2.0 mL/kg)耳缘静脉麻醉。常规消毒,铺巾。取股骨中段前外侧3.0 cm纵行切口,钝性分离股骨外侧肌群,通过肌间隙显露股骨干。取股骨干中段前外侧1 cm×2 cm骨面作为置板区域,以磨钻小心打磨骨皮质至出现新鲜点状渗血。使用1.0 mm克氏针在打磨区均匀钻孔,微孔间距2~3 mm。将3D打印多孔钛板放置妥当后使用2.4 mm锁定螺钉固定。反复冲洗切口,逐层缝合。
-
术后第4、16周,分别从3组实验兔中选取3只,处死后取出股骨并仔细剥离周围软组织,用于大体观察、影像学观察。依次对股骨干标本进行4%多聚甲醛组织固定、梯度乙醇脱水、二甲苯透明、甲基丙烯酸甲酯包埋处理,然后对标本进行切割和打磨抛光处理, 切片厚度控制在50 μm左右,最后进行VG染色处理,用于组织学观察。
-
大体观察:观察植入钛板周围是否有骨膜包被,钛板与股骨干之间是否有骨组织生成以及与钛板结合情况。影像学观察:通过X线片观察植入钛板位置及钛板周围是否有骨痂形成,钛板与骨面之间是否有缝隙。组织学观察:镜下观察各标本多孔结构中是否有新生骨组织,并采集图像信息(每个标本2张),用Image-pro Plus 6.0软件对新生骨组织面积进行半定量分析,计算新生骨形成率,新生骨形成率=新生骨组织面积/多孔钛板内部可供骨长入孔隙的总面积×100%。
-
采用单因素方差分析和q检验。
1.1. 实验材料
1.2. 方法
1.2.1. 兔股骨干模型的重建及打印
1.2.2. 多孔金属钛板的设计
1.2.3. 实验动物建模
1.2.4. 实验标本取材与处理
1.3. 观察指标
1.4. 统计学方法
-
各标本植入的多孔钛板均固定在位,未见明显移位现象。术后4周,各组标本钛板周围及与骨面间可见纤维组织,未见明显骨痂形成。术后16周,各组标本钛板和骨面之间有新生骨组织形成连接,钛板周围有较多的骨痂形成,个别钛板被骨痂包裹(见图 3)。
-
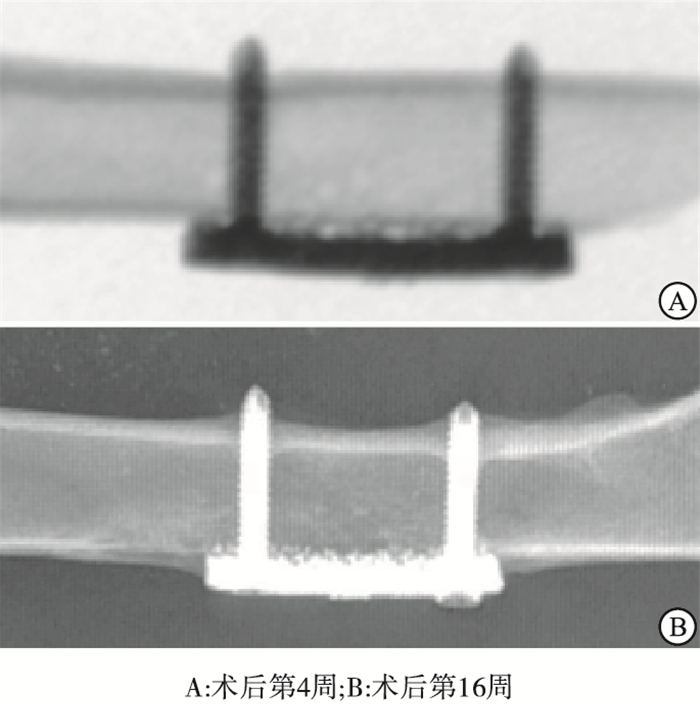
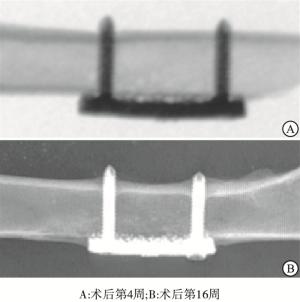
X线片观察显示,各组钛板均固定在位。术后4周多孔钛板与骨质之间无明显骨痂形成,部分钛板与骨面之间存在有一定间隙。术后16周钛板周围均有不同程度的骨痂形成,部分钛板被骨痂包绕,钛板与骨面结合紧密,未见明显间隙(见图 4)。
-
术后16周,A组实验兔钛板边缘可见骨组织长入,但由于孔隙率过低,各孔隙之间空间较小,孔隙内部骨长入欠佳;B组可见一定量的新骨生成并长入孔隙之中,但骨组织与钛板之间存在一定缝隙,骨-植入物界面骨结合不良,易发生微动影响远期稳定性;C组钛板孔隙间空间适宜,各孔隙互有联通,新生骨组织长入孔隙内部,长入密度较高,且与钛板结合紧密(见图 5)。3组新生骨形成率间差异有统计学意义(P<0.01),其中C组均明显高于A组和B组(P<0.01),B组亦明显高于A组(P<0.01)(见表 1)。
分组 新生骨形成率/% F P MS组内 A组 13.69±1.86 B组 22.87±2.99** 79.40 <0.01 10.383 C组 36.96±4.33**## q检验:与A组比较**P<0.01;与B组比较##P<0.01
2.1. 实验兔大体观察结果
2.2. 实验兔影像学观察结果
2.3. 组织学观察
-
钛金属具有密度低、强度高、耐腐蚀、生物力学性能和生物相容性好的特点,是目前已知生物亲和力最好的金属。其弹性模量较其他金属更接近人体骨骼的弹性模量,成为目前骨科内植物材料的优先选择[4]。常规钛植入物表面光滑,内部无孔隙结构,只能单纯被纤维组织包裹,主要依赖与宿主骨骼之间的摩擦力和来自螺钉钛缆的第三方固定来保持稳定,无法完成骨长入过程[5]。为解决这一问题,模拟骨小梁结构的三维孔隙结构被引入钛植入物的制作之中,利用3D打印技术特殊的制作工艺,将钛合金制造成多孔植入物。3D打印技术可精确控制多孔结构的孔隙率、孔径、空间排列等参数。
多孔钛金属植入物有以下优点[6-8]:(1)可以通过孔径和孔隙率的调节来改变弹性模量,减少应力屏蔽效应,实现植入物和宿主骨之间的力学性能的匹配;(2)增加材料比表面积和材料-骨接触面积,促进骨整合;(3)孔隙的存在可保持细胞形态,为成骨细胞提供黏附和增殖空间,有利于骨长入;(4)孔隙之间相互联通,有利于氧分和营养物质的运输以及代谢物的交换。近年多孔钛金属植入物从实验到临床亦得到了多维度应用。YANG等[9]对实验绵羊行3D打印多孔钛合金自稳人工颈椎置换术,组织学观察显示骨组织可以顺利长入人工椎体孔隙内部,并能维持颈椎的稳定性。ARANDA等[10]利用3D打印制造个体化多孔钛胸骨,用于因胸壁肿瘤行胸骨切除后的修复,效果得到认可。XU等[3]为一例尤文肉瘤患儿行全球首例3D打印多孔人工定制枢椎的置换,术后恢复良好。
骨长入是指骨组织通过植入物表面或内部的微孔或孔隙结构与植入物完成骨性结合。影响多孔植入物骨长入的因素很多,如孔隙率、孔径、孔隙连通性、材料的生物相容性、生物活性、应力刺激等[11]。关于适合骨长入的最佳孔隙率,一般认为高孔隙率不影响细胞黏附,可提供利于营养物质和氧流动、运输的空间,促进细胞增殖和骨髓间充质干细胞成血管化,有利于骨长入;孔隙率过小则导致孔隙空间狭窄,缺少有效联通,细胞增殖到一定范围会产生“接触抑制”[12]。CHENG等[13]在制作的15%、38%、70%孔隙率多孔钛支架上培养细胞后发现高孔隙率较低孔隙率更利于细胞分化。王雷等[14]将兔骨髓间充质干细胞加入40%和70%孔隙率的多孔钛合金支架中,培养72 h后发现70%孔隙率较40%孔隙率更适宜细胞的黏附和增殖。有学者[15]通过观察翻修的生物型多孔假体,发现多孔结构里新生骨占比不到10%,并且孔隙内骨质交联较少,考虑这种现象可能与假体的孔隙率过低有关。
但并不是孔隙率越高越有利于骨长入。LV等[16]通过向多孔金属假体中植入骨髓间充质干细胞,发现73%孔隙率组的细胞活性和分化情况均优于81%孔隙率组。周鹏等[17]分别将3种孔径和孔隙率均不相同的3D打印钛合金假体植入兔股骨髓腔,结果表明,262 μm孔径、68.1%孔隙率的假体在骨体积分数、组织矿物密度和最大拔出力等方面均高于558 μm孔径、79.2%孔隙率和753 μm孔径、89.0%孔隙率假体。另外,也有少数文献称中、高孔隙率对骨长入的影响无显著差异,如KUJALA等[18]构建鼠股骨缺损模型并植入47%和66%孔隙率的镍钛合金支架,结果显示2组孔隙率对成骨的影响并无统计学差异。
本实验结果显示,75%孔隙率的钛板孔隙中新骨形成率达到36.96%,明显高于其他2组的新骨形成率。分析其原因可能是75%孔隙率比例合适,孔隙间连通性高,为成骨细胞提供足够生长空间,更易于氧和营养物质运输;多孔钛板为贴附融合,孔隙结构厚度有限,35%孔隙率过于紧密,孔隙间无法形成有效交通,导致新骨组织局限在外围,无法深入其中。
目前,对于像本实验这种以多孔植入物在颅颈表面、长骨骨皮质骨表面直接贴合形式的骨长入研究鲜有文献报道。因此,本研究具有一定的创新性,可以为此类骨长入研究提供参考。但本实验也存在一些局限和不足之处:(1)相关研究不多,缺乏大量相关文献的支持,结果评价标准有限也缺乏统一;(2)样本量不多,长入时间有限,未能观察更长时间骨长入情况。因此,在后续的实验中还需要针对存在的问题进行完善。
综上所述,本研究发现3D打印多孔钛板的孔隙结构,可以允许新生骨长入;不同孔隙率大小,影响3D打印钛金属植入物的新生骨长入效果;35%、55%、75%三种孔隙率中,75%孔隙率较其他两组较低孔隙率更有利于骨长入;75%孔隙率3D打印多孔钛板可与经适当处理的皮质骨表面实现良好的骨性融合。实验具有一定的科学性和创新性,可为3D打印个体化定制枕颈融合器在骨性融合方面提供参考。










 DownLoad:
DownLoad: