-
GABA能神经元通过编码动作电位发放模式在维持大脑功能内环境的稳态中起着关键作用。研究[1]表明大脑皮层GABA能神经元的内在特性包括阈值(Vts-Vr)和绝对不应期(absolute refractory period, ARP)共同调控着神经编码模式。神经元动作电位产生过程中有着抑制成分如后超极化(AHP)通过降低电压门控钠通道介导的阈值电位和ARP来调控着动作电位的编码[2]。钾通道能以不同的方式调节神经元兴奋性和编程,例如,快速后超极化(fAHP)、慢速后超极化(sAHP)[3-6]。然而,这些AHP成分如何对大脑皮层GABA能神经元内在特性产生影响,目前尚不清楚。
大脑皮层GABA能神经元接受兴奋性突触输入与抑制性突触输入的时间和空间上的总和效应[7-8],代数和形成了膜电位的波动,正向电流促使膜电位去极化,负向电流使膜电位发生超极化。GABA能神经元整合兴奋性和抑制性突触输入后会产生的不同类型的电位波动[fAHP、sAHP、快速后去极化(fADP)、完全复极和未完全复极],它们对于内在特性和编码的影响目前还不明确。
为了解决上述问题,我们利用出生15~20 d的小鼠,使用全细胞记录来探讨不同类型电位波动对神经元内在特性和神经编码的影响,为进一步了解抑制性神经元的内在特性变化及对神经编码的影响提供实验基础和理论依据。
HTML
-
Tris-GTP(Sigma); Mg2+-ATP(Sigma); Hepes(Sigma); EGTA(Sigma); Na2phosphocreatine(Sigma); Kclu(Sigma)。人工脑脊液(ACSF)的制备:10 mmol/L dextrose,124 mmol/L NaCl,3 mmol/L KCl,1.3 mmol/L NaH2PO4,0.5 mmol/L CaCl2和4 mmol/L MgSO4,5 mmol/L Hepes,26 mmol/L NaHCO3。充分氧合后备用。
电极标准液的制备:0.5 mmol/L EGTA;4 mmol/L NaCl;10 mmol/L Hepes;150 mmol/L葡萄糖酸钾;4 mmol/L Mg2+-ATP;1 mmol/L Tris-GTP;调整pH至7.35,渗透压295~305 mOsmol,保持电极阻抗为5~6 MΩ。
-
选用出生15~20 d的FVB-Tg(Gad GFP)45 704 Swn/J小鼠,麻醉后快速断头取脑,置于备用的ACSF中,制成每片400 μm厚度的脑片3~5片,将制作的脑片移至充分氧合的ACSF中,环境温度设置为35 ℃,离体培养1 h。取孵育的脑片1片,将其放置于IR-DIC显微镜下,温浴槽温度设置为35 ℃,灌流液灌流速度不超过每分钟50滴。运用全细胞记录方法对脑片中大脑皮层GABA能神经元进行电生理学记录,使用pClamp 10软件将电生理学数据进行记录。
-
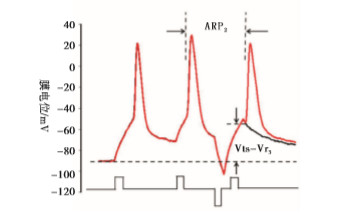
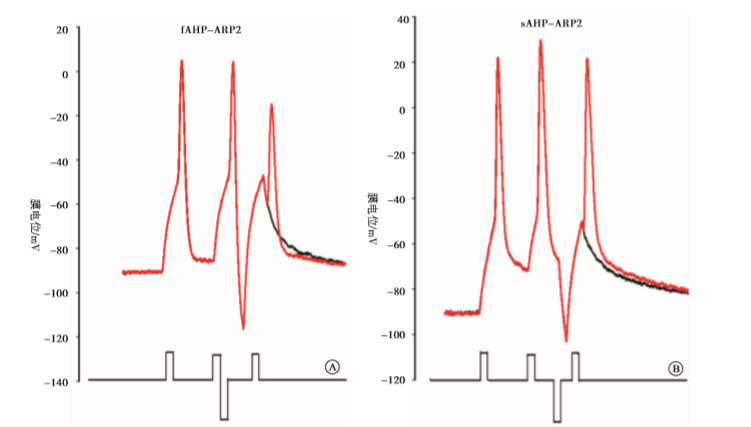
实验分4组:fAHP组、sAHP组、动作电位复极组、fADP组。fAHP组:在给予标准的去极化刺激强度后,立即给予不同强度的超极化刺激,测得相应的ARP和Vts-Vr,实验模式见图 1A;sAHP组:在给予标准的去极化刺激强度后,在一定时间间隔后(3 ms)给予不同强度的超极化刺激,测得相应的ARP和Vts-Vr,实验模式见图 1B;动作电位复极组:在紧跟着动作电位后给予不同标准化去极化刺激,即为阈刺激的0、0.25、0.5、0.75倍,以模拟动作电位未完全和完全复极化。fADP组:在紧跟着动作电位后给予标准化去极化刺激,即为阈刺激的1.25、1.5倍,以模拟动作电位的fADP。
-
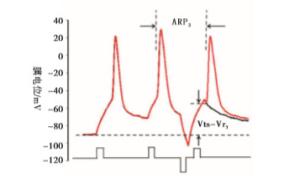
内在特性指标包括动作电位的能障(Vts-Vr)和动作电位的ARP[9-14]。Vts为细胞膜去极化达到的某一临界膜电位,此时能够促使Na+通道大量开放,它的测量如图 2所示[9-10],Vr表示静息膜电位。能障(Vts-Vr)测量见图 2。ARP的测量:以阈强度,刺激时程为3 ms的去极化电流引导细胞产生一个动作电位,设置20 ms后以相同的刺激产生下一个动作电位,改变两个动作电位的时间间隔,当下一个动作电位刚好产生时的时间间隔为ARP[10]。测量方法见图 2。
-
通过阈强度去极化电流引导动作电位产生,在标准化超极化刺激强度下,即为阈刺激的(-0.5、-1、-1.5、-2、-2.5)倍时,量化fAHP与sAHP的ARP2值并进行比较。通过阈强度去极化电流引导动作电位产生,在标准化超极化刺激强度下,即为阈刺激的(-0.5、-1、-1.5、-2、-2.5)倍时,量化fAHP和sAHP的Vts-Vr3值并进行比较。在动作电位产生后未复极之前立刻给予阈刺激的0.25、0.5、0.75倍的刺激,以模拟动作电位未完全复极化,量化ARP2的值;动作电位完全复极后测量ARP2的值,并与前2组进行比较。通过阈强度去极化电流引导动作电位产生,在标准化超极化刺激强度下,在紧跟着动作电位后给予标准化去极化刺激,即为阈刺激的1.25、1.5倍,以模拟动作电位的fADP,量化ARP2的值。
-
采用t检验。
1.1. 主要试剂及制备
1.2. 实验方法
1.3. 实验分组
1.4. ARP、Vts-Vr的测量方法
1.5. 电生理实验步骤
1.6. 统计学方法
-
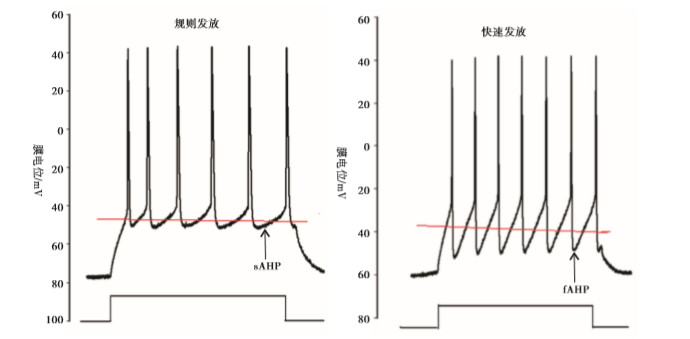
大脑皮层GABA能神经元的规则发放表现为等高的峰幅度,逐渐延长的动作电位峰间距(inter-spike intervals, ISI)和较明显的频率适应性,而快发放表现为等高的峰幅度,较短的几乎相等的ISI和很小的频率适应性(见图 3)。
-
通过阈强度去极化电流引导动作电位产生,在标准化超极化刺激强度下,即为阈刺激的(-0.5、-1、-1.5、-2、-2.5)倍时,量化ARP2值并进行比较。fAHP和sAHP相比,fAHP影响下的动作电位的ARP2较短(P < 0.01)(见表 1)。
分组 n -0.5 -1 -1.5 -2 -2.5 fAHP 9 4.43±0.04 4.32±0.04 4.35±0.06 4.21±0.06 4.35±0.07 sAHP 15 5.48±0.07 7.04±0.85 7.98±0.61 11.23±0.81 15.63±1.01 t — 40.94 9.51 17.64 25.73 33.16 P — < 0.01 < 0.01 < 0.01 < 0.01 < 0.01 -
通过阈强度去极化电流引导动作电位产生,在标准化超极化刺激强度下,即为阈刺激的(-0.5、-1、-1.5、-2、-2.5)倍时,量化Vts-Vr3值并进行比较。fAHP和sAHP相比,fAHP影响下的动作电位的Vts-Vr3较高(P < 0.01)(见表 2)。
分组 n -0.5 -1 -1.5 -2 -2.5 fAHP 9 38.32±0.31 39.07±0.46 37.4±0.6 37.07±0.62 35.49±0.73 sAHP 15 32.35±0.21 34.75±0.55 35.19±0.52 35.88±0.60 35.63±0.78 t — 56.41 17.94 9.52 4.65 0.44 P — < 0.01 < 0.01 < 0.01 < 0.01 >0.05 -
fAHP的影响如前所述,即阈刺激的(-2.5、-2、-1.5、-1、-0.5)倍ARP2值分别为(4.43±0.04)、(4.32±0.04)、(4.35±0.06)、(4.21±0.06)、(4.35±0.07)ms(n=12)。动作电位完全复极,即为阈刺激的0倍,测得的ARP2的值为:(3.97±0.05) ms(n=12)。在动作电位产生后未复极之前立刻给予阈刺激的0.25、0.5、0.75倍的刺激,以模拟动作电位未完全复极化,量化ARP2的值,分别是:(3.72±0.04)、(3.81±0.06)和(3.82±0.05) ms(n=12)。综上,在阈刺激的(-2.5、-2、-1.5、-1、-0.5、0、0.25、0.5、0.75)倍下测得ARP2值进行比较,当为阈强度0.25倍时,ARP2最短(P < 0.05)。
-
fAHP的影响如前所述,即阈刺激的(-2.5、-2、-1.5、-1、-0.5)倍ARP2值分别为(4.43±0.04)、(4.32±0.04)、(4.35±0.06)、(4.21±0.06)、(4.35±0.07) ms(n=12)。动作电位完全复极,即为阈刺激的0倍,测得的ARP2的值为:(3.97±0.05) ms(n=12)。通过阈强度去极化电流引导动作电位产生,在标准化超极化刺激强度下,在紧跟着动作电位后给予标准化去极化刺激,即为阈刺激的1.25、1.5倍,以模拟动作电位的fADP,量化ARP2的值,分别是(4.07±0.06)和(4.26±0.08) ms(n=12)。综上,在阈刺激的(-2.5、-2、-1.5、-1、-0.5、0、1.25、1.5)倍下测得ARP2值进行比较,ARP2在动作电位完全复极时最短(P < 0.05)。
2.1. 大脑皮层两类GABA能神经元发放模式的比较
2.2. fAHP、sAHP对ARP的影响
2.3. fAHP、sAHP对Vts-Vr的影响
2.4. fAHP和动作电位不同复极化对ARP的影响
2.5. fAHP和fADP对ARP的影响
-
本实验以实验方法模拟fAHP、sAHP、fADP、动作电位完全复极和未完全复极,量化小鼠大脑皮层GABA能神经元连续动作电位内在特性,包括:ARP2和Vts-Vr3,它们是较好地体现神经元的内在特性的指标[12-14]。我们发现fAHP与sAHP相比较,fAHP可以通过缩短ARP2的同时增加Vts-Vr3以诱导第三个动作电位的产生。ARP变短可以使后一个动作电位波峰向前一个波峰进行移动,从而使在固定的刺激时间内增加动作电位发放的容量,动作电位发放容量增加导致神经元的快发放,其发放动作电位的能力增加;相反,sAHP通过延长ARP,诱导其规则发放。阈值电位越低,对兴奋性突触输入的敏感性越高,意味着其兴奋性越高。值得注意的是,fAHP增加了阈电位,而sAHP降低了阈电位。一般认为抑制性突触的兴奋性驱动,膜电位是向远离发放动作电位的阈值移动的[15],我们的研究表明由fAHP诱导的更高阈电位也支持这一观点。电压门控钠通道(VGSCs)的动力学决定了动作电位的阈电位水平[2],实验结果表明,施加sAHP相较于fAHP其激活的VGSC更多,所以对兴奋性突触输入更为敏感,更容易启动动作电位的发放。实验结果表明,fAHP可缩短ARP,而sAHP可降低阈电位,不仅能抑制神经元活动,而且能增强神经元的放电能力。由于动作电位的ARP和阈电位由VGSCs的动力学控制[16-19],上述现象的产生表明,由K通道介导的fAHP和sAHP[2]可能是通过引起VGSC动力学的可塑性变化而影响神经元的内在特性和编码的,相关工作需要进一步进行探讨。
在fAHP、静息电位水平和不完全复极条件下,复极电位水平越接近静息电位,ARP就越短。如前所述,较短的ARP增加神经元发放容量,对于刺激的敏感性提高,兴奋性增加。大脑皮层中大多数神经元其膜电位往往处于阈下状态,使神经网络对兴奋性突触输入更为敏感[20]。我们的结果表明,阈下电位通过缩短ARP来提高神经元的兴奋性,神经元内在特性的变化可能是由接近静止电位水平的膜电位复极引起的。在fAHP、静息电位水平和fADP中,随着膜电位的波动,ARP2随fADP的降低而变短,在接近静息膜电位处变得最短。fADP由钙激活非选择性电流介导,调节大脑皮层神经元动作电位早期的快发放[21]。由于ARP由VGSC的动力学控制,施加fAHP与fADP相比,在静息膜电位下有更多VGSC被激活,从而缩短ARP以提高兴奋性。一个神经元可与多个神经元之间建立突触联系,既可以是兴奋性的,也可以是抑制性的,它们在时间空间上进行总和,引起神经元膜电位的变化,如fAHP、sAHP、fADP、不完全复极等。我们的研究结果证明多突触传入总和效应可以通过影响突触后神经元的内在特性,进而影响其编码,以及产生动作电位的能力,这与以前的研究[22]一致。
综上所述,大脑皮层GABA能神经元在整合突触输入后,当膜电位波动时其内在特性发生可塑性变化,不同的输入模式通过影响大脑皮层GABA能神经元动作电位的内在特性,从而调控GABA能神经元对输入信号的编程能力。这些变化使神经元对突触信号输入更为敏感,使中枢神经系统[23-24]对机体活动起到更加稳定的调节。







 DownLoad:
DownLoad: