-
肝细胞性肝癌(HCC)发病率居世界第五位, 因癌死亡原因的第三位, 每年有超过60万病人死于该病[1], 目前HCC在我国死亡率居各种肿瘤死亡率的第二位[2]。随着诊断手段的增加及临床筛选的重视, 发现早期HCC并进行有效的治疗逐步成为可能。而肝活检标本内早期高分化肝细胞肝癌(HDHCC)与异型增生结节(DN)的鉴别诊断一直困扰着病理学界, 尽管肝癌国际合作组织提供了较为详细的组织学诊断标准, 但实际工作中阅片医生的诊断标准的尺度把握参差不齐, 主观性因素常常导致最终诊断的偏差。因此, 在组织形态学基础上探寻能区分两者的相对特异的免疫组织化学(免疫组化)标记或联合标记成为研究热点。本文将探究磷脂酰肌醇蛋白聚糖-3(GPC-3)、CD10、CD147、CD138免疫组化标记在HDHCC与DN中的表达情况, 寻求区分HDHCC与DN的客观诊断依据。
HTML
-
选择2013年4月至2018年9月我院经病理确诊且资料完整的HDHCC病例76例, 术前均未经放疗、化疗。76例中男48例, 女28例, 年龄32~81岁。挑取带有癌组织且癌旁DN的蜡块, 行免疫组化GPC-3、CD10、CD147、CD138标记。
-
鼠抗人单克隆抗体CD10(克隆号:56C6)、CD147(克隆号:1A6)、CD138(克隆号:MI15)及SP试剂盒均购自北京中衫生物技术公司, 鼠抗人单抗GPC-3(克隆号:MAXIM001)购自福州迈新生物技术开发有限公司。
-
组织抗原修复:标本经10%中性甲醛溶液固定, 常规脱水透明、石蜡包埋, 免疫组化防脱片切片厚度4 μm。常规脱蜡水化放于蒸馏水中备用。采用EDTA抗原修复液经高压锅抗原修复。全自动免疫组化染色机染色:将修复过的组织切片插入机器染色卡槽, 自动运行染色步骤(内源性过氧化物酶阻断, 一抗孵育, 二抗孵育, DAB显色)。
-
GPC-3和CD147细胞质内出现黄色或棕黄色颗粒为阳性。CD10和CD138细胞膜或细胞质均可着色, 以细胞膜为主。阳性表达评价标准:在高倍显微镜下(×400倍)随机选取5个视野, 观察阳性细胞占比率。阴性(-):占比率 < 10%;弱阳性(+):占比率10%~25%;中等阳性(2+):占比率26%~50%;强阳性(3+):占比率>50%。
-
采用χ2检验。
1.1. 标本来源
1.2. 试剂
1.3. 方法
1.4. 结果判定
1.5. 统计学方法
-
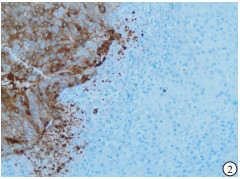
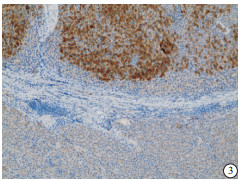
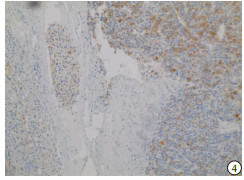
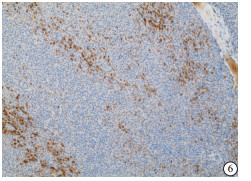
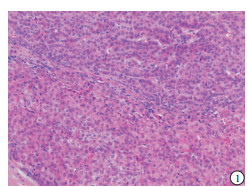
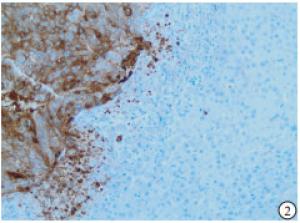
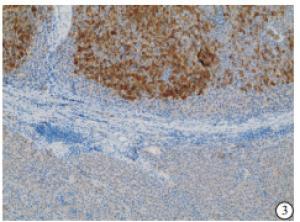
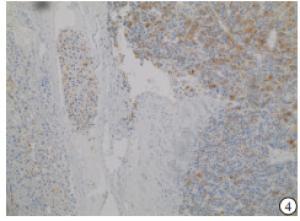
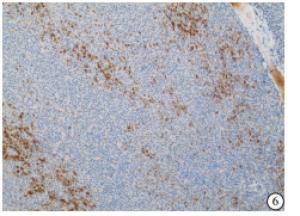
HE染色示:HDHCC与HD组织形态学非常接近, 鉴别相当困难, 尤其是活检标本更加困难(见图 1)。免疫组化示:HDHCC中GPC-3、CD10、CD147较DN内高表达, 且DN内表达常常杂乱不均(见图 2~5); 而CD138在DN中高表达, HDHCC弱表达或不表达(见图 6)。
-
HDHCC和DN内GPC-3、CD10、CD147和CD138的表达差异均有统计学意义(P < 0.01)(见表 1)。
分组 n GPC-3 CD10 CD147 CD138 + - + - + - + - HDHCC 76 66 10 58 18 62 14 27 49 DN 76 31 45 28 48 24 52 44 32 χ2 - 34.90 24.10 38.67 7.64 P - < 0.01 < 0.01 < 0.01 < 0.01 -
GPC-3、CD10、CD147和CD138联合检测对于HDHCC的敏感度为96.05%(70/76), 特异度为52.63%(见表 2), 说明4种标记物联合检测能明显提高HDHCC的检出率, 但特异度并不高, 需要紧密结合组织形态学改变来区分HDHCC与DN。
指标 灵敏度 特异度 GPC-3+CD10 68(89.47) 48(63.15) GPC-3+CD10+CD147 72(94.73) 43(56.57) GPC-3+CD10+CD147+CD138 73(96.05) 40(52.63)
2.1. 电镜观察结果
2.2. GPC-3、CD10、CD147和CD138在HDHCC与DN内的表达
2.3. GPC-3、CD10、CD147和CD138对HDHCC的联合检测
-
肝癌尤其是HDHCC若能早发现、早诊断并积极治疗可获得极佳的预后。医学影像技术的进步使得更小的、更早的肝内结节得以发现, 但结节的性质最终需要病理检查来确诊。病理临床实践中对HDHCC与DN的鉴别一直是学界热点和难点。鉴别的难点在于两者在形态学上比较接近或类似, 实际工作中往往凭借阅片人的主观经验来区分。因此, 寻求相对客观的单独或联合免疫组化标记作为鉴别辅助手段一直方兴未艾。近年来, 国内外针对HCC采用免疫组化标记及基因检测等手段, 以期揭示从肝硬化到异型增生, 再到早期HDHCC过程中具有预警价值的客观指标, 一些零散的、单个或少数联合的癌变相关抗原的免疫组化标记辅助鉴别诊断研究被报道[3-8]。受相关报道启发, 本研究对HDHCC标本行GPC-3、CD10、CD147、CD138联合标记, 与瘤周DN相对照来观察两者表达有无差异性。
GPC-3属硫酸类肝素糖蛋白聚糖家族成员, 其一般在人体胚胎期表达, 成人中则仅在胎盘组织内高表达, 在正常肝脏组织中无GPC-3表达[9-10]。本研究显示GPC-3蛋白在HDHCC组织中高表达, 癌周DN内低表达或不表达, 与文献[5, 11]报道结果一致。CD10属金属蛋白酶家族成员, 为Ⅱ型单链穿膜糖蛋白, 参与细胞生长、成熟、分化、增殖、迁移等生理调节过程, 在肿瘤的发生发展中起重要生物学效应。本研究发现CD10在HDHCC细胞质及沿肝窦呈小管状、分支状着色, 且癌组织较周围异型增生组织高表达, 与KADOTA等[12]报道一致, 对肝癌与异型增生具鉴别诊断价值。CD147又称细胞外基质金属蛋白酶诱导因子, 属免疫球蛋白超家族成员。CD147广泛存在于多种细胞中, 不仅参与胚胎发育、生精过程、淋巴细胞发育, 还在肿瘤侵袭转化、心脏功能衰竭、肝组织损伤、病毒侵入细胞等多种生理病理过程中起重要作用[13-15]。近年来, 有关CD147在致瘤作用、诱导免疫逃逸及靶向治疗方面的研究取得了较大进展[16-19]。本研究显示CD147在HDHCC组织内的表达高于癌周DN, 提示CD147参与肝癌发生过程且对HDHCC与HD的鉴别诊断具有一定价值。CD138属整合素家族成员, 为细胞表面跨膜乙酰肝素蛋白聚糖分子, 起连接细胞表面介导与细胞外配体(如瘤细胞周围细胞上的血小板内皮细胞黏附因子等受体)作用, 可维持细胞黏附稳定性, 与细胞迁移密切相关[20]。本研究显示肝细胞癌CD138表达缺失, 与文献[21]报道肝细胞癌变过程中可能与细胞-基质间黏附作用降低, 细胞失去生长接触抑制功能而无限增殖有关。故CD138标记亦可以用于HDHCC与HD的鉴别诊断。
综上所述, GPC-3、CD147、CD10及CD138在HDHCC及癌旁DN中的表达差异明显, 联合标记可明显提升两者鉴别的敏感度, 且特异度较高。在肝活检组织形态学基础上进行GPC-3、CD147、CD10及CD138联合检测可作为客观辅助手段用于HDHCC与HD的鉴别诊断。







 DownLoad:
DownLoad: