-
染色体病是一种常见的遗传病, 它是由于机体内外环境因素导致的染色体数目异常或结构畸变[1]。染色体结构异常但表型正常的携带者后续临床表现可能包括流产、死胎、新生儿死亡、新生儿智力低下、胎儿畸形等, 且出现畸形儿或新生儿智力低下的可能性达到100%[2-4]。提高人口素质, 降低出生缺陷是我国的民生工程, 因此各个地区已经广泛开展了染色体分析技术, 其中外周血淋巴细胞染色体核型分析已成为优生优育、产前诊断、辅助生殖的常规检测项目; 同时也是研究染色体与临床疾病关系的重要手段[5-6]。由于外周血淋巴细胞染色体核型分析实验操作过程比较繁琐, 影响因素很多, 各实验室的染色体培养、制备效果也有所不同。如何稳定获得形态良好、长度适宜、分散较好、足量的中期分裂象染色体依然是遗传学实验室亟待解决的一个问题。本实验室通过对外周血淋巴细胞染色体培养这个环节进行改良, 取得了不错的效果。现将改良的培养方法作一报道。
HTML
-
选取2018年3月来本院进行遗传咨询并行外周血染色体核型分析的病人共40例, 每例病人均取肝素抗凝静脉血2份, 1份为改良组, 1份为传统方法对照组。
-
CO2培养箱为上海力申公司生产, 水浴箱为上海博讯公司产品, 低速离心机由安徽中科中佳生产, 超净工作台为苏州净化公司产品, CX41显微镜为Olympus公司产品, 淋巴细胞培养基和秋水仙素为广州达晖公司生产, 胰蛋白酶为美国Ampesco公司生产, 姬姆萨染液为深圳亿立方公司产品, 甲醇、冰醋酸、KCl、NaCl、KH2PO4、Na2HPO4等均为国产试剂。
-
改良组将肝素抗凝血2000r/min离心7min后, 用无菌滴管吸取上层的血浆和白细胞层, 然后再混匀取0.3mL加人淋巴细胞培养基中培养68~72h(注意无菌操作)。对照组肝素抗凝血0.5mL加入淋巴细胞培养基中培养68~72h。
-
收获前1.5h加20mg/L秋水仙素100μL, 将培养的细胞移入15mL刻度离心管中, 2000r/min离心7min后, 弃上清液, 加入已预温的5.587g/L氯化钾溶液7mL, 吹打均匀后置于37℃水浴箱中温育30min, 取出离心管加入新鲜配制的固定液(甲醇:冰乙酸=3:1)1mL[7], 轻轻混匀后放置室温5min(预固定)。2000r/min离心7min后, 弃上清液, 再加入固定液7mL, 固定10min后, 重复上述步骤再固定一次。离心后弃上清液, 再加入1.5~2mL的固定液, 调成合适浓度的细胞悬液, 以看不见明显混浊为宜。在温度25~28℃, 湿度50%~60%的条件下, 将细胞悬液滴在预冷的洁净的湿片上。将滴好的玻片放在烤箱70℃烘烤3h, 自然冷却至37℃。
-
将老化的玻片按常规G显带操作, 并参照国际染色体G显带标准对染色体进行分析。将0.01g胰酶加入到50mL pH 7.2的缓冲液中; 姬姆萨染液4mL加磷酸盐缓冲液至50mL; 0.9%氯化钠溶液备用(用于胰酶消化后清洗)。以上试剂均需预温至37℃备用。染色前需预染, 最佳时间为40s。
-
不合格标本:染色体长度较短, 染色体带纹不清, 但可以辨认出2号、4号、6号、12号、18号、20号、22号染色体特征性带型; 合格标本:可以清楚地辨认2号、4号、6号、12号、18号、20号、22号染色体特征性带纹, 其中染色体可分析且长度较理想的核型5%~25%;最佳标本:在合格标本上, 染色体可分析且长度理想的核型≥ 25%。
-
采用组内配对χ2检验。
1.1. 材料
1.1.1. 标本来源
1.1.2. 仪器与试剂
1.2. 培养方法
1.3. 染色体制备
1.4. G显带和标准
1.5. 带型效果判断
1.6. 统计学方法
-
改良组标本全部培养成功, 成功率为100%。对照组培养成功39例, 成功率为97.5%。改良组与对照组的培养成功率差异无统计学意义(P>0.05)。每份标本观察100个分裂象, 计算≥ 400条带分裂象所占的百分比, 结果显示, 改良组高于对照组(P < 0.01)(见表 1)。
分组 n 培养成功 ≥ 400条带 改良组 40 40(100.0) 1 980(49.5) 对照组 40 39(97.5) 1 300(32.5) P - >0.05* < 0.01* *示组内配对χ2检验 -
改良组带型的合格率与最佳率均高于对照组(P < 0.05和P < 0.01) (见表 2)。
分组 n 最佳 合格 不合格 合格率/% 最佳率/分% 改良组 40 30 9 1 97.5 75.0 对照组 40 4 27 9 77.5 10.0 P - - - - < 0.05* < 0.01* *示组内配对χ2检验 -
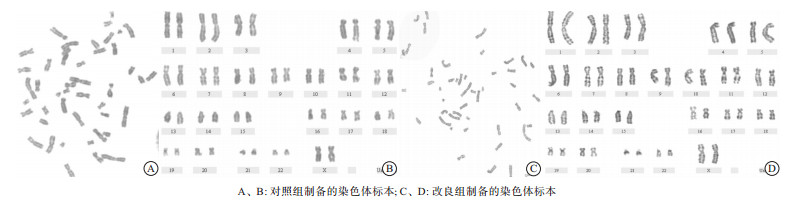
与对照组相比, 改良后的外周血染色体分散度好, 分裂相多, 长度更长, 带型清晰(见图 1)。改良后染色体长度更长, 分散度更好, 显示带型增多。
2.1. 2组染色体培养成功率与分裂象显带的比较
2.2. 2组染色体培养带型合格率与最佳率的比较
2.3. 2组染色体培养分散度、分裂象、长度等比较
-
随着科学技术的发展, 人们对保健知识及优生优育意识的不断增强, 染色体分析技术在优生优育和遗传疾病的诊断得到了广泛的应用[8-9], 而制备出高质量的染色体核型则是细胞遗传学研究的基础。传统的外周血淋巴细胞培养是一个复杂、繁琐并且重复的过程, 它包括细胞培养、细胞低渗、细胞固定、细胞滴片、细胞老化和显带等步骤, 实验过程中若有一个操作环节出现失误, 就极有可能导致实验的失败, 所以在本实验中, 细胞培养环节至关重要。本文就是针对细胞培养环节进行了改良。参照其他实验室或文献提供的方法, 外周血淋巴细胞培养的时间一般为68~72h, 用肝素抗凝全血进行培养。本实验室采用血浆和白细胞层进行培养。结果显示:改良培养方法不仅成功率得到提高, 而且染色体长度、带型也得到了很大程度的改善。其中, 染色体长度、带型的改善可能是红细胞在生长过程中无氧酵解, 不断释放CO2并产酸, 使培养基pH发生变化, 影响了淋巴细胞的活性, 从而干扰其分裂。而本试验是去除了红细胞的干扰, 保留了血浆, 从而保证了淋巴细胞在分裂过程中有充足的营养, 所以改良后培养的染色体比较长。因而染色体越长则带纹越清晰, 分辨率越高, 从而获得了大量的400条带以上的分裂象。
总之, 通过处理外周血标本然后进行淋巴细胞染色体培养, 不仅得到了高质量的中期染色体核型, 还有助于后续的显带和核型分析, 从而提高诊断结果的准确性, 有较好的应用和推广价值。







 DownLoad:
DownLoad: