-
心房颤动(atrial fibrillation,AF)简称房颤,主要表现为心脏迅速而又不规则地跳动,是临床上最常见的一种心律失常,其发病率及致死率居高不下,危害性堪比恶性肿瘤,严重影响病人的生命质量[1]。心房纤维化的特征在于心肌成纤维细胞异常增殖所致的心肌细胞外基质(extracellular matrix, ECM)的异常积累[2],它作为AF发生的触发器,可引起心房电传导异常,更易形成折返,有利于AF的发作和维持,是AF发生、发展的病理基础[3]。二十二碳六烯酸(docosahexaenoic acid, DHA)属于ω-3多不饱和脂肪酸家族中的重要成员,在膳食鱼类中广泛存在。研究[4]表明,增加多不饱和脂肪酸的摄入可有效降低冠心病和心血管疾病事件发生的风险。最新研究[5]也证实,多不饱和脂肪酸可发挥抗AF的作用。丝裂原激活蛋白激酶磷酸酶(mitogen activates protein kinase phosphatase, MKP-1)是一种苏氨酸/酪氨酸磷酸酯酶,可调控丝裂原激活蛋白激酶(mitogen activated protein kinase,MAPKs)及P38磷酸化状态。正常情况下,MAPKs和MKP-1处于动态平衡状态,这种平衡状态在决定细胞生存或凋亡方面起重要作用[6]。另有研究[7]发现, MAPK通路可介导成纤维细胞的增殖和迁移。而DHA是否通过影响P38 MAPK通路发挥抗AF的作用尚未见报道。本研究观察DHA对大鼠心房纤维化的影响,并探讨该作用是否与调控P38 MAPK通路介导的胶原表达有关。
HTML
-
SD雄性大鼠80只,体质量(220±20)g,由蚌埠医学院实验动物中心提供。氯化乙酰胆碱购于Sigma公司,单克隆鼠抗P38、P-P38、MKP-1抗体及β-actin抗体均购于Santa Cruz公司,单克隆二抗购自武汉博士德公司;大鼠Ⅰ型、Ⅲ型胶原ELISA试剂盒购于上海慧嘉生物公司,PBS购于Hyclone公司,其余试剂均为国产分析纯级别。MedLab生物信号采集处理系统(U/4C501H)购于南京美易科技有限公司,低温高速离心机(5810R)于德国Eppendor公司购买,膜片钳相关仪器设备由美国Axon公司提供,免疫印迹配套设备购于美国BIO-RAD公司。
-
雄性SD大鼠适应性喂养1周后,尾静脉注射[8]66 μg/mL乙酰胆碱与50 mg/mL氯化钙混合液,心电图(标准Ⅱ导联)全程记录。剔除乙酰胆碱-氯化钙混合液不敏感大鼠,最终选择80只,随机分为对照组(CON组)、对照DHA处理组(DHA组)、AF组和AF+DHA处理组(DHA+AF组),每组20只。
-
AF模型组连续3 d尾静脉注射乙酰胆碱-氯化钙混合液,每天给药1次,Ⅱ导心电图显示P波消失、出现f波的典型AF心电图视为模型复制成功[8]。
-
第4天开始,CON组给予0.9%氯化钠溶液,DHA组给予DHA溶液(10 mg/mL)和0.9%氯化钠溶液,AF组给予0.9%氯化钠溶液和乙酰胆碱-氯化钙混合液,DHA+AF组给予DHA溶液和乙酰胆碱-氯化钙混合液,给药方式均为尾静脉注射,给药剂量均为0.1 mL/100 g,每天给药1次,连续14 d。
-
参照陈春林团队的建模方法[8],实验第3、4、10、17天记录给药前后心电图,以P波消失、f波出现为AF发生标志;窦性心律恢复、P波复现、f波消失为AF终止标志,AF发生至终止所持续的时间即为AF持续时间。
-
实验第18天,脱颈法处死SD大鼠后,迅速开胸取出心脏,剪取左心房肌条固定于持续通以混合气体(95%O2+5%CO2)的含有K-H液的浴槽,调节前负荷为1g,稳定40 min,采用连续双刺激法测定心房肌ERP。
-
采用改良的酶解法分离出大鼠心房肌细胞,选取富有活力、静止、杆状、横纹清晰的细胞,应用电流钳方法引发心肌细胞动作电位,全细胞膜片钳技术记录复极50%时的动作电位时程(APD50)、复极90%时的动作电位时程(APD90)。
-
剪取的心房组织剪碎后置于研钵中,加入1 mL 4 ℃预冷的PBS快速研磨至未见明显的组织块,2 000 min,离心10 min后取上清,按照试剂盒中的方法,使用酶标仪在450 nm波长下检测并记录各组OD值。
-
提取心房组织总蛋白,使用BCA法蛋白定量后,经SDS-PAGE凝胶电泳、湿转法转膜、5%脱脂奶粉封闭、分别用单克隆抗体P38(1∶500)、P- P38(1∶500)、MKP-1(1∶500)4 ℃孵育过夜、二抗(1∶1 000)室温孵育1 h后ECL显色照相。采用Bio-Rad quantity one对目的条带进行半定量分析。
-
采用t检验、方差分析和q检验。
1.1. 实验动物与试剂
1.2. AF动物模型制备与分组
1.2.1. 大鼠初筛与分组
1.2.2. AF模型复制
1.2.3. 分组干预处理
1.3. AF持续时间测定
1.4. 大鼠心房有效不应期(ERP)测定
1.5. 大鼠心房肌细胞动作电位时程(APD)测定
1.6. ELISA检测大鼠心房肌组织胶原表达
1.7. 心房肌MAPK信号通路蛋白表达
1.8. 统计学方法
-
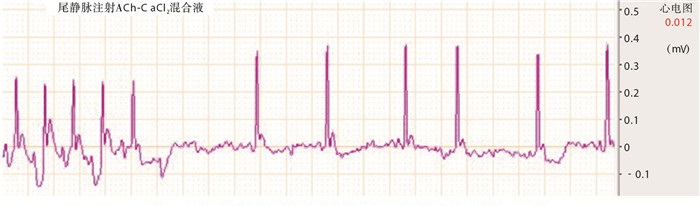
大鼠尾静脉注射乙酰胆碱-氯化钙混合液1~2 s后即出现典型的AF心电图(见图 1)。
-
CON组和DHA组尾静脉注射0.9%氯化钠溶液,给药前后心电图无明显变化,未见典型AF心电图。AF组和DHA+AF组通过观察心电图,结果显示随着实验时间的增加,AF大鼠的AF持续时间逐渐延长,实验第4、10、17天,与AF组相比,DHA+AF组大鼠AF持续时间明显缩短(P<0.05~P<0.01)(见表 1)。
分组 第3天 第4天 第10天 第17天 AF组 6.20±1.6 11.8±3.1 28.8±2.4 41.0±2.7 DHA+AF组 7.40±1.1 9.20±1.8 16.6±2.5 17.6±1.7 t 1.95 2.29 11.13 23.19 P >0.05 < 0.05 < 0.01 < 0.01 -
与CON组大鼠相比,AF组ERP明显缩短,DHA组及DHA+AF ERP组延长(P<0.01);与AF组相比,DHA+AF组大鼠ERP明显延长(P<0.01)(见表 2)。
分组 ERP/ms F P MS组内 CON组 80.0±2.6## 424.99 < 0.01 6.055 AF组 60.3 ±1.5** DHA组 95.7±1.5**## DHA+AF组 83.0±3.6**##▲▲ q检验:与CON组比较**P<0.01;与AF组比较##P<0.01;与DHA组比较▲▲P<0.01 -
与CON组相比,AF组大鼠心房肌细胞APD50和APD90明显缩短(P<0.01),DHA组与DHA+AF组延长(P<0.01);与AF组相比,DHA+AF组大鼠心房肌细胞APD50和APD90延长(P<0.01)(见表 3、4)。
分组 APD50 F P MS组内 CON组 0.99±0.08##▲▲ 86.74 < 0.01 0.006 AF组 1.22 ±0.10**## DHA组 0.88±0.04**▲▲ DHA+AF组 1.08±0.07**##▲▲ q检验:与CON组比较**P<0.01;与AF组比较##P<0.01;与DHA组比较▲▲P<0.01 分组 APD90 F P MS组内 CON组 0.99±0.07##▲▲ 129.50 < 0.01 0.006 AF组 0.81±0.06**##▲▲ DHA组 1.20 ±0.08**## DHA+AF组 1.15±0.09**## q检验:与CON组比较**P<0.01;与AF组比较##P<0.01;与DHA组比较▲▲P<0.01 -
与CON组相比,AF组Ⅰ型、Ⅲ型胶原表达明显增高(P<0.01),DHA组Ⅰ型、Ⅲ型胶原表达明显降低(P<0.01);与AF组相比,DHA、DHA+AF组Ⅰ型、Ⅲ型胶原表达均明显降低(P<0.01)(见表 5、6)。
分组 Ⅰ型胶原水平/(μg/L) F P MS组内 CON组 40.6±8.8##▲▲ 85.58 < 0.01 31.133 AF组 63.3±4.8**▲▲ DHA组 27.2±3.8**## DHA+AF组 43.9±3.1##▲▲ q检验:与CON组比较**P<0.01;与AF组比较##P<0.01;与DHA组比较▲▲P<0.01 分组 Ⅲ型胶原水平(μg/L) F P MS组内 CON组 4.89±0.85## 12.58 < 0.01 1.841 AF组 6.97±0.91**▲▲ DHA组 3.66 ±2.17## DHA+AF组 4.67±1.05## q检验:与CON组比较**P<0.01;与AF组比较##P<0.01;与DHA组比较▲▲P<0.01 -
各组P38表达无明显变化,与CON组相比,DHA组P-P38表达明显下降,MKP-1表达明显增加(P<0.01);AF组P-P38表达明显增高,MKP-1表达明显降低(P<0.01);与AF组相比,DHA+AF组P-P38表达明显下降,MKP-1表达明显增加(P<0.01)(见表 7、8)。
分组 P-P38/P38 F P MS组内 CON组 0.60±0.05## 3.03 < 0.05 0.054 AF组 0.82±0.04**▲▲ DHA组 0.56 ±0.46## DHA+AF组 0.71±0.03**##▲▲ q检验:与CON组比较**P<0.01;与AF组比较##P<0.01;与DHA组比较▲▲P<0.01 项目分组 MKP-1/β-actin F P MS组内 CON组 0.70±0.06##▲▲ 125.90 < 0.01 0.003 AF组 0.46±0.04**▲▲ DHA组 0.87 ±0.06**## DHA+AF组 0.75±0.05##▲▲ q检验:与CON组比较**P<0.01;与AF组比较##P<0.01;与DHA组比较▲▲P<0.01
2.1. 大鼠AF心电图
2.2. DHA对大鼠AF持续时间的影响
2.3. DHA对大鼠心房肌ERP影响
2.4. DHA对大鼠心房肌细胞APD影响
2.5. DHA对大鼠心房肌胶原表达影响
2.6. DHA对大鼠心房肌MAPK信号通路蛋白表达影响
-
AF是一种进行性、自我持续性心律失常,AF及其并发症成为一个日益严重的临床问题[9]。本实验通过复制AF模型发现:与CON组相比,AF组大鼠AF持续时间随实验时间增加而逐渐延长,心房ERP、APD50及APD90明显缩短,心房组织P-P38蛋白、Ⅰ型及Ⅲ型胶原表达水平明显升高,MKP-1表达下降;DHA干预组AF持续时间较AF组明显缩短,以实验第10、17天尤为显著,在有效延长心房ERP、APD50及APD90的同时还下调了P-P38、Ⅰ型及Ⅲ型胶原表达水平,而MKP-1水平较前增加。这些结果表明,DHA可能通过调控P38 MAPK通路发挥抗AF作用。
AF的一个显著特征是心房结构重构,而心房纤维化的形成是导致AF病人心房结构重构的主要因素之一。研究证实[10],ECM参与了心房重塑过程,心脏成纤维细胞分泌的胶原在ECM的形成中起着关键作用。多种胶原在ECM中表达并参与促纤维化反应,Ⅰ型胶原约占心肌胶原的80%~90%,Ⅲ型胶原则与AF复发率独立相关,当组织受到刺激时,心肌成纤维细胞过度增殖,各型胶原合成和分泌增加,降解减少,发生胶原过度聚集,即ECM重构[10]。本研究发现,与CON组相比,AF组大鼠Ⅰ型、Ⅲ型胶原表达明显增高,提示AF的发生伴随着心肌纤维化程度加重;与AF组相比,给予DHA治疗后,大鼠心房Ⅰ型、Ⅲ型胶原表达显著降低,提示DHA可通过改善胶原成分比例减轻纤维化,抑制心房重构。
2009年的一项研究[11]表明,鱼油补充剂中的DHA不仅有助于健康人群的心血管疾病预防,而且可减少心脏病病人的心脏事件和死亡率。近年来,DHA在防治AF方面的作用日益受到关注。流行病学随访研究发现,膳食鱼类的人群与AF的低发病率密切相关[12], RAMADEEN等[13]在狗的AF模型中的研究发现DHA可以降低心房纤维化的程度进而抑制AF的发生、发展。本实验发现,通过DHA干预可显著缩短AF持续时间,还可有效延长心房ERP、APD50及APD90,降低Ⅰ型、Ⅲ型胶原表达,与RAMADEEN等的结果一致,提示DHA可有效减轻AF大鼠心肌纤维化。
MAPKs是细胞内的一类丝氨酸和(或)苏氨酸激酶,是细胞应激和炎症反应的主要信号通路,MAPK的活性受MKP负调控,MKP-1缺乏可导致应激反应中P38 MAPK通路活化增强[14]。在小鼠肌少症肥胖模型中发现,多不饱和脂肪酸对P38 MAPK信号通路所介导的骨骼肌细胞凋亡和细胞分化减少有保护作用[15]。相关研究[16]显示,多不饱和脂肪酸在保护心肌缺血再灌注损伤中具MAPK-1依赖性。有实验[17]证明,P38 MAPK的激活可显著增加AF的易感性。本实验发现,与CON组相比,DHA处理组P-P38表达明显下降,MKP-1表达明显增加(P<0.05);与AF组相比,DHA+AF组P-P38表达明显下降,MKP-1表达明显增加。心房肌组织P38 MAPK活性的逐渐增高与MKP-1表达相对下降呈显著负相关关系,这与文献[14]中报道的一致。本研究发现,在各组大鼠中,P-P38蛋白的表达与Ⅰ型、Ⅲ型胶原表达趋势一致,提示DHA不仅降低了P38的表达,还可降低Ⅰ型、Ⅲ型胶原表达,结合我们课题组前期研究[18],本实验中DHA可改善AF心房肌纤维化,其机制可能与激活P38 MAPK通路密切相关。
值得注意的是,心房重构包括了结构重构和电重构,后者[19]主要指AF时节律快而不规则的心房激动造成的心房组织电生理特性的改变,常表现为ERP和APD一致性缩短。本实验中,与CON组大鼠相比,AF组大鼠ERP、APD50及APD90均表现为同步缩短,使用DHA干预后ERP、APD50及APD90均得到一致有效延长,提示DHA可能还发挥对抗AF大鼠的心房电重构作用,其具体作用机制有待下一步深入研究。关于P38 MAPK通路与DHA的摄入鲜有报道,本研究初步证明DHA具有抗心房纤维化作用,该作用的实现可能与P38 MAPK通路调控的胶原表达有关,将为AF病人临床治疗的研究提供新的方向,但具体机制仍需进一步探讨。







 DownLoad:
DownLoad: