-
原发性肝癌为临床常见消化系统肿瘤之一,其恶性程度高,近年来其发病率及死亡率仍有升高趋势[1-2]。目前,肝动脉化疗栓塞是无法手术治疗的原发性肝癌的首选治疗方案,临床应用广泛、效果良好[3]。但原发性巨块型肝癌的肿瘤直径较大,采用肝动脉化疗栓塞虽能取得一定疗效,但肿瘤供血血管无法完全栓塞坏死,残存肿瘤可以通过侧支循环继续增殖、转移,从而影响病人预后[4]。近年来,随着栓塞材料和技术的发展,我国自主研发的CalliSpheres载药微球逐渐应用于肝癌栓塞治疗中,国内已有大量研究指出CalliSpheres载药微球栓塞是治疗原发性肝癌的安全、有效方法[5-6]。但其在原发性巨块型肝癌中的应用效果少有研究报道。鉴于此,本研究将CalliSpheres载药微球栓塞应用于我院原发性巨块型肝癌病人,旨在探究其疗效及对肿瘤标志物、肝功能的影响。现作报道。
HTML
-
选取我院2017年1月至2019年1月原发性巨块型肝癌病人80例作为研究对象,按照随机数字表法分为2组,各40例。2组性别、年龄、体质量、肿瘤数目、Child肝功能分级[7]、合并症,差异均无统计学意义(P>0.05)(见表 1),具有可比性。
分组 n 男 女 年龄(x±s)/岁 体质量(x±s)/kg 肿瘤数目 Child肝功能分级 合并症 单发 多发 A级 B级 高血压 冠心病 高血脂 观察组 40 25 15 56.81±6.90 66.42±9.78 36(90.00) 4(10.00) 18(45.00) 22(55.00) 5(12.50) 3(7.50) 6(15.00) 对照组 40 22 18 55.74±7.35 67.55±8.72 34(85.00) 6(15.00) 15(37.50) 25(62.50) 4(10.00) 6(15.00) 3(7.50) χ2 — 0.46 0.67* 0.55* 0.46 0.46 0.00 0.50 0.50 P — >0.05 >0.05 >0.05 >0.05 >0.05 >0.05 >0.05 >0.05 *示t值 -
纳入标准:(1)符合原发性巨块型肝癌诊断标准[8];(2)Child肝功能分级A级或B级;(3)首次发病;(4)未接受相关治疗;(5)认知功能良好,无沟通交流障碍;(6)病人知晓本研究,已签署同意书。排除标准:(1)合并其他恶性肿瘤者;(2)继发性肝癌病人;(3)肝癌晚期病人;(4)肿瘤发生肝外转移者;(5)血液系统疾病病人;(6)糖尿病病人;(7)严重心脑肺肾功能不全者;(8)伴有门静脉血栓疾病者。
-
对照组:给予碘油经导管动脉化疗栓塞,采用改良Seldinger法经股动脉穿刺置管,栓塞前静脉注射地塞米松类药物减轻化疗不良反应,经导管将85~100 mg/m2奥沙利铂(海南锦瑞制药有限公司,国药准字H20143023)注入,随后将10~20 mL超液态罂粟乙碘油与20~60 mg吡柔比星(深圳万乐药业有限公司,国药准字H10930105)混悬乳剂缓慢注入供血动脉,栓塞后可适量追加明胶海绵颗粒。
观察组:给予CalliSpheres载药微球经导管动脉化疗栓塞,CalliSpheres载药微球直径为100~300 μm(江苏恒瑞伽俐生生物医药科技有限公司,规格1 g),股动脉穿刺置管及导管超选择插管、奥沙利铂注入等操作与对照组相同,微球载药(用20 mL注射器抽取微球后与3~4 mL注射用水溶解的60~80 mg吡柔吡星溶液均匀混合,每5 min摇晃1次,载药时间20~30 min),配置完成后,用1 mL注射器以1 mL/min的速度将CalliSpheres载药微球脉冲式注入肿瘤供血血管,进行动脉化疗栓塞,直至对比剂流速停滞时停止栓塞,观察5 min后再次造影,栓塞至肿瘤染色完全消失。
-
(1) 2组术后1个月、3个月临床效果,参照《原发性肝癌诊疗规范(2017年版)》[9]制定疗效评估标准,分为疾病进展(disease progression,PD)、疾病稳定(stable disease,SD)、部分缓解(partial remission,PR)、完全缓解(complete remission,CR),疾病缓解率=(PR+CR)/总例数×100%,疾病控制率=(SD+PR+CR)/总例数×100%。(2)2组术前、术后1个月、3个月血清肿瘤标志物水平,包括糖类抗原199(Carbohydrate antigen 199,CA199)、甲胎蛋白(alpha fetoprotein,AFP)、E-钙黏蛋白(E-cadherin,EC),采集病人空腹静脉血6 mL,以3 500 r/min转速离心处理5 min后取血清,置于-70 ℃冷藏室内待检,取血清标本,采用化学发光法检测血清CA199水平,试剂盒购自天津博奥赛斯生物科技有限公司[批准文号:国食药监械(准)字2012第3400961号,规格:96人份/盒],采用胶乳增强免疫比浊法检测血清AFP及EC水平,试剂盒购自北京利德曼生化股份有限公司[批准文号:国食药监械(准)字2013第3401971号,规格:R1:1×80 mL R2:1×16 mL],检测操作均由专业人员严格按照试剂盒说明书进行。(3)2组术前、术后1个月、3个月肝功能指标水平[天冬氨酸氨基转移酶(aspartate aminotransferase,AST)、丙氨酸氨基转移酶(alanine aminotransferase,ALT)]、总胆红素(total bilirubin,TBIL)、白蛋白(albumin,Alb),取血清标本,采用全自动干式生化分析仪(南京大树生物医疗技术股份有限公司,批准文号:苏械注准20172400293)检测上述指标水平。(4)2组术后并发症发生率,包括疼痛、肝脓肿、发热、化疗相关性恶心呕吐(chemotherapy-induced nausea and vomiting,CINV),疼痛采用视觉模拟评分法[10]评估,总分0~10分,得分>4分纳入疼痛评定范围;CINV分级[11]:未出现恶心、呕吐为0级;轻微恶心、无呕吐为Ⅰ级;暂时性的恶心、呕吐为Ⅱ级;明显恶心、呕吐,需要治疗为Ⅲ级;难以控制的恶心、呕吐为Ⅳ级。(5)治疗后随访1年,统计2组1年生存率。
-
采用t检验、方差分析和Dunnett-t检验、χ2检验、Ridit检验、卡普兰-迈耶(Kaplan-Meier,KM)曲线分析和Log-Rank检验。
1.1. 一般资料
1.2. 入组标准
1.3. 方法
1.4. 观察指标
1.5. 统计学方法
-
2组术后1个月疾病控制率与疾病缓解率差异均无统计学意义(P>0.05);术后3个月观察组疾病控制率和疾病缓解率高于对照组(P < 0.05)(见表 2)。
分组 n CR PR SD PD 疾病缓解率 疾病控制率 术后1个月 观察组 40 1(2.50) 24(60.00) 12(30.00) 3(7.50) 25(62.50) 37(92.50) 对照组 40 0(0.00) 22(55.00) 14(35.00) 4(10.00) 22(55.00) 36(90.00) χ2 — — — — — 0.46 0.00 P — — — — — >0.05 >0.05 术后3个月 观察组 40 0(0.00) 23(57.50) 10(25.00) 7(17.50) 23(57.50) 33(82.50) 对照组 40 0(0.00) 12(30.00) 12(30.00) 16(40.00) 12(30.00) 24(60.00) χ2 — — — — — 6.15 4.94 P — — — — — < 0.05 < 0.05 -
术前,2组血清CA199、AFP、EC水平差异均无统计学意义(P>0.05);术后1个月、3个月,2组血清CA199、AFP、EC均低于术前(P < 0.01),且观察组低于对照组(P < 0.01)(见表 3)。
分组 n 术前 术后1个月 术后3个月 F P MS组内 CA199/(U/L) 观察组 40 84.11±10.24 36.37±6.31** 48.92±8.74** 332.48 < 0.01 73.687 对照组 40 83.56±9.78 47.51±8.06** 61.13±9.93** 153.40 < 0.01 86.406 t — 0.25 6.88 5.84 — — — P — >0.05 < 0.01 < 0.01 — — — AFP/(ng/mL) 观察组 40 188.62±30.49 78.14±21.52** 116.44±26.08**△△ 182.18 < 0.01 690.972 对照组 40 185.20±32.07 113.36±24.89** 153.37±28.14** 63.73 < 0.01 813.286 t — 0.49 6.77 6.09 — — — P — >0.05 < 0.01 < 0.01 — — — EC/(ng/mL) 观察组 40 2 716.55±223.41 1 814.43±164.06** 2 096.77±202.38** 216.95 < 0.01 39 261.792 对照组 40 2 712.39±208.76 2 142.59±181.92** 2 385.36±210.49** 81.10 < 0.01 40 327.221 t — 0.09 8.47 6.25 — — — P — >0.05 < 0.01 < 0.01 — — — Dunnett-t检验:与术前组比较**P < 0.01 -
术前、术后1个月,2组血清AST、ALT、TBIL、Alb差异均无统计学意义(P>0.05);术后3个月,观察组血清AST、ALT、TBIL低于对照组(P < 0.01),Alb高于对照组(P < 0.01)(见表 4)。
分组 n 术前 术后1个月 术后3个月 AST/(U/L) 观察组 40 82.23±12.27 76.59±10.34 83.04±12.71 对照组 40 80.64±13.03 78.02±11.16 127.58±15.49 t — 0.56 0.59 14.06 P — >0.05 >0.05 < 0.01 ALT/(U/L) 观察组 40 68.14±10.41 45.58±8.13 65.20±11.42 对照组 40 67.28±10.06 47.23±8.55 81.19±15.03 t — 0.38 0.88 5.36 P — >0.05 >0.05 < 0.01 TBIL/(μmol/L) 观察组 40 50.06±7.14 47.15±8.02 71.11±13.34 对照组 40 50.23±7.53 48.46±8.39 105.37±17.52 t — 0.10 0.71 9.84 P — >0.05 >0.05 < 0.01 Alb/(g/L) 观察组 40 35.78±5.29 33.48±6.13 32.24±5.08 对照组 40 36.01±6.05 32.95±5.75 27.13±4.61 t — 0.18 0.40 4.71 P — >0.05 >0.05 < 0.01 -
观察组疼痛、肝脓肿发生率与对照组差异均无统计学意义(P>0.05);观察组发热发生率、CINV分级均低于对照组低(P < 0.05和P < 0.01)(见表 5)。
分组 n 疼痛 肝脓肿 发热 CINV分级 0 Ⅰ Ⅱ Ⅲ 观察组 40 7(17.50) 0(0.00) 12(30.00) 8(20.00) 21(52.50) 7(17.50) 4(10.00) 对照组 40 9(22.50) 1(2.50) 23(57.50) 3(7.50) 9(22.50) 18(45.00) 10(25.00) χ2 — 0.31 — 6.15 3.33 P — >0.05 >0.05* < 0.05 < 0.01 *示确切概率法 -
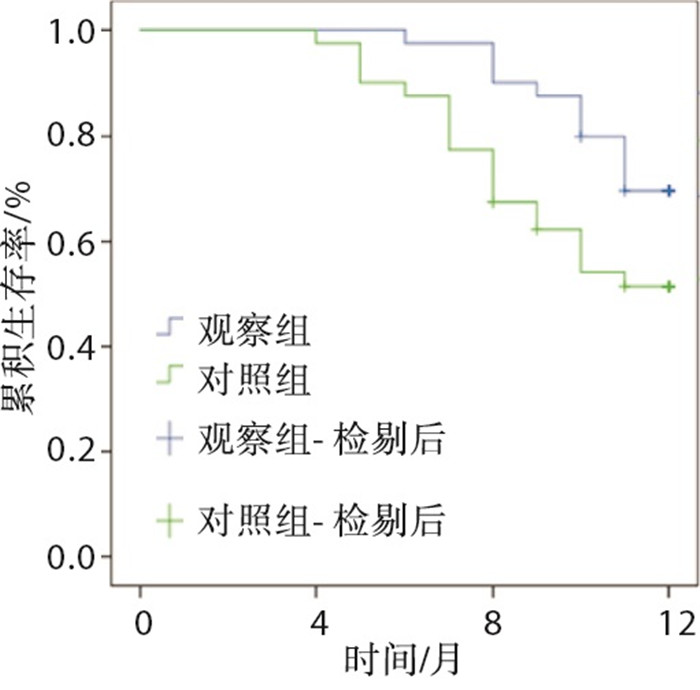
随访1年,观察组失访2例,对照组失访3例。观察组术后1年生存28例,死亡10例,生存率为73.68%(28/38),对照组生存19例,死亡18例,生存率为51.35%(19/37);观察组术后1年生存率高于对照组(χ2=4.00,P < 0.05)(见图 1)。
-
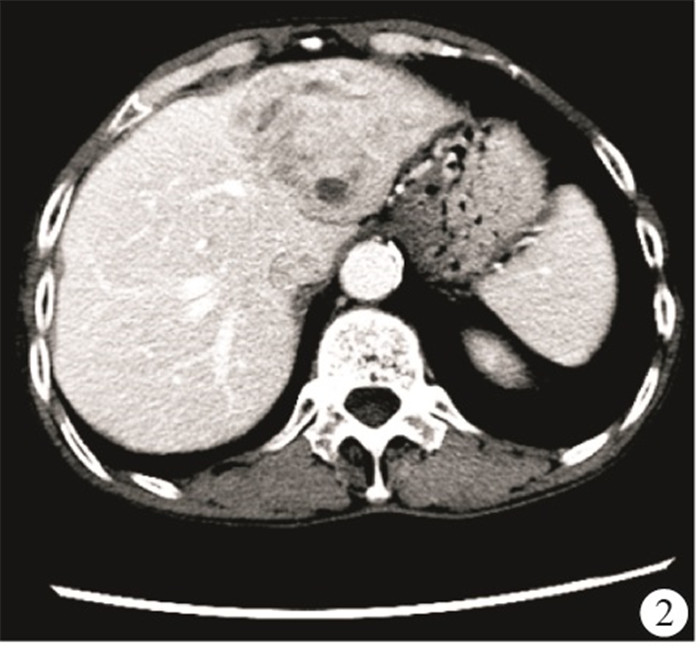
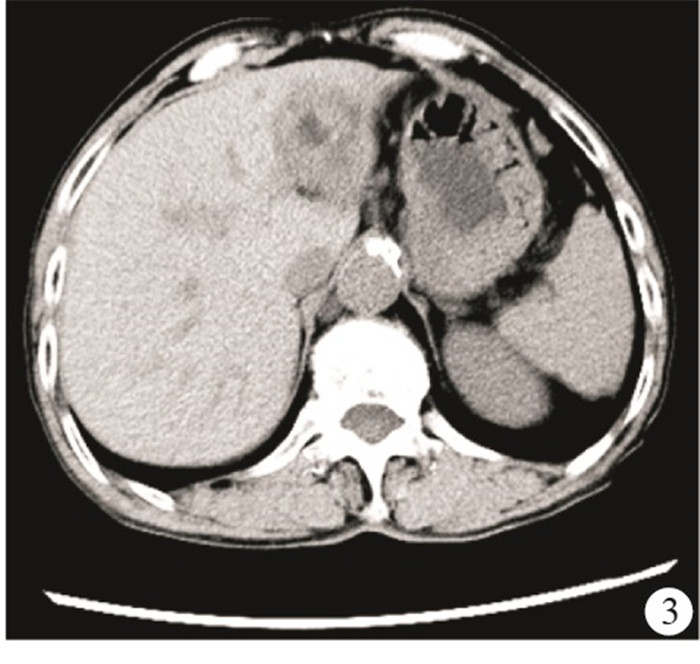
病人男,71岁,腹痛腹胀1个月入院,经CT、超声及肿瘤标志物等检查证实为原发性巨块型肝癌,临床分期为Ⅰb期,术前腹部增强CT显示肝脏出现巨大包块,动脉期肿瘤不均匀强化显著(见图 2)。接受CalliSpheres载药微球经导管动脉化疗栓塞治疗,术后1个月经腹部增强CT复查显示肿瘤病灶明显缩小,临床效果评价为完全缓解(见图 3)。
2.1. 临床效果
2.2. 肿瘤标志物
2.3. 肝功能指标
2.4. 术后并发症
2.5. 1年生存率
2.6. 典型病例
-
巨块型肝癌常为单发性癌块,也可由多个结节汇集成一大块,肿瘤直径较大,往往不能进行手术治疗[12]。我国2011肝癌治疗指南将巨块型肝癌列为肝动脉化疗栓塞治疗的适应证,明确指出分期为Ⅰb~Ⅲb均可行肝动脉化疗栓塞治疗[13]。
肝动脉化疗栓塞的传统方法为碘油经导管动脉化疗栓塞,为尽量提高抗肿瘤效果,栓塞过程中常需加大碘油剂量,碘油剂量增加可加重肝功能损伤,甚至能引发肝功能衰竭,严重影响病人预后[14]。目前,国产CalliSpheres载药微球在原发性肝癌肝动脉化疗栓塞治疗中的作用越来越受临床重视,国内学者葛世堂等[15]研究指出,国产CalliSpheres载药微球栓塞治疗不可切除肝癌是安全可行的,近期临床疗效较好,值得临床推广应用。周永祥等[16]将CalliSpheres载药微球栓塞治疗应用于高龄肝癌病人,同样取得了良好临床效果。我们此次研究将CalliSpheres载药微球栓塞应用于原发性巨块型肝癌的治疗中,发现其术后1个月的疗效与常规碘油
经导管动脉化疗栓塞无明显差异,但术后3个月的疾病缓解率、疾病控制率均明显高于碘油经导管动脉化疗栓塞。分析其原因在于:碘油经导管动脉化疗栓塞将碘油、化疗药混合成混悬乳剂进行栓塞治疗,混悬乳剂稳定性欠佳,缺乏化疗药持续缓释的效果,近期疗效良好,但随着时间延长,其化疗作用逐渐减弱;而CalliSpheres载药微球栓塞则不同,CalliSpheres载药微球作为新型可装载化疗药物的栓塞材料,其药物缓释效应良好,通过使装载化疗药物的微球在肿瘤血管内部均匀分布,可持久、缓慢释放化疗药物,从而达到长期、持续的抗肿瘤效果。相关研究证实,CalliSpheres载药微球可有效延长抗肿瘤时间,增强栓塞效果[17]。因此CalliSpheres载药微球栓塞能取得持久、有效的治疗效果。CA199、AFP、EC为临床常见肿瘤标志物,临床已有大量研究证实上述指标可作为评估原发性肝癌诊断、病情评估、疗效评估的重要指标[18-19]。本研究发现,采用CalliSpheres载药微球栓塞治疗能进一步下调原发性巨块型肝癌病人血清CA199、AFP、EC水平,充分证实CalliSpheres载药微球栓塞在原发性巨块型肝癌治疗方面同样能取得显著治疗效果。
肝动脉化疗栓塞的主要治疗机制在于化疗药物对肿瘤细胞的灭活作用,在发挥抗肿瘤作用的同时,对正常肝细胞及周围组织同样具有危害性[20]。相关报道[21]显示,中国原发性肝癌肝动脉化疗栓塞术后恶心呕吐、局部疼痛、发热等并发症发生率均较高,是影响病人治疗依从性及预后效果的主要因素之一[21]。本研究发现,采用CalliSpheres载药微球栓塞治疗,能显著降低化疗药物对原发性巨块型肝癌病人血清AST、ALT及Alb水平的影响,有助于保护病人肝功能,且能有效降低发热发生率,减轻恶心呕吐程度。由于碘油经导管动脉化疗栓塞是在短时间内大量释放化疗药物,对正常肝细胞的损伤程度高,而CalliSpheres载药微球栓塞可缓慢、持久释放化疗药物,可有效避免上述缺陷,减轻肝细胞损伤,从而有效降低并发症发生率。本研究结果还显示,CalliSpheres载药微球栓塞治疗1年后,原发性巨块型肝癌的生存率为73.68%,明显高于碘油经导管动脉化疗栓塞治疗的病人,提示CalliSpheres载药微球栓塞在改善原发性巨块型肝癌病人预后方面具有明显优势。
综上可知,CalliSpheres载药微球栓塞治疗原发性巨块型肝癌的疗效显著、安全性高,能有效减轻对病人肝功能的影响,提高病人1年生存率,具有较高临床应用价值。相信随着国产CalliSpheres载药微球栓塞治疗的不断推广应用,其在原发性肝癌治疗中将发挥重要作用。








 DownLoad:
DownLoad: