-
我国肺癌发病率与死亡率逐年上升,目前肺癌发病机制尚未完全阐明,长链非编码RNA(LncRNA)是一种长度超过220 nt的非编码RNA,LncRNA与微小RNA(miRNA)之间存在相互作用,其基因序列上含有多个miRNA的结合位点,并可通过调控miRNA转录过程从而参与肿瘤发生及发展过程,已知敲低LncRNA XIST通过激活miR-335/SOD2/ROS信号途径而抑制非小细胞肺癌的发展[1]。LncRNA Gm15290通过与肿瘤抑制因子miR-615-5p直接相互作用从而促进肺癌细胞的增殖和侵袭[2]。LncRNA MALAT1通过调节miR-124/STAT3轴促进非小细胞肺癌的发展[3]。LncRNA TUC338通过激活MAPK途径促进肺癌的侵袭[4]。LncRNA LRRC75A-AS1通过调控BAALC从而促进三阴性乳腺癌细胞的增殖和侵袭[5]。但LRRC75A-AS1在肺癌中的表达及其可能作用机制尚未阐明。靶基因预测显示miR-22-3p与LRRC75A-AS1存在结合位点,miR-22-3p在非小细胞肺癌中表达水平降低,LncRNA NNTAS1通过调节miR-22-3p/YAP1轴促进非小细胞肺癌的进展[6]。但LRRC75A-AS1/miR-22-3p分子轴在肺癌发生过程中的作用机制尚未阐明。因此,本研究主要探讨LRRC75A-AS1对肺癌细胞增殖、凋亡、迁移的影响及其对miR-22-3p的调控作用。
HTML
-
收集2018年3月至2019年2月期间于本院接受手术治疗的43例肺癌病人的肺癌组织及癌旁组织(>5 cm)标本,所有病人均经病理诊断为肺腺癌,TNM分期:Ⅰ期22例、Ⅱ期18例、Ⅲ期3例。其中男23例,女20例,年龄55~68岁,平均(62.38±6.32)岁。本研究经本院伦理委员会批准,所有病人知情且签署同意书。人肺癌细胞A549购自上海匹拓生物科技有限公司;DMEM培养基购自美国Gibco;胎牛血清购自美国Hyclone;Lipofectamine2000、凋亡检测试剂与MTT试剂购自北京索莱宝;Trizol试剂购自美国Invitrogen;反转录试剂盒、SYBR Green试剂盒购自北京天根生化;si-NC、si-LRRC75A-AS1、miR-NC、miR-22-3p mimics、si-LRRC75A-AS1与anti-miR-NC、si-LRRC75A-AS1与miR-22-3p inhibitor购自广州锐博生物。
-
A549细胞(2.5×105个/毫升)接种于96孔板(100微升/孔),按照Lipofectamine2000转染试剂说明书分别将si-NC、si-LRRC75A-AS1、si-LRRC75A-AS1与anti-miR-NC、si-LRRC75A-AS1与miR-22-3p inhibitor转染入A549细胞,分别记为si-NC组、si-LRRC75A-AS1组、si-LRRC75A-AS1+anti-miR-NC组、si-LRRC75A-AS1+miR-22-3p inhibitor组。
-
采用Trizol法提取癌旁组织、肺癌组织与各组A549细胞总RNA,反转录体系:5×Reaction Buffer 2 μL,10×King RT Buffer 2 μL,FastKing RT Enzyme Mix 1 μL,FQ-RT Primer Mix 2 μL,RNA(2 μg),RNase-Free ddH2O补足体系至20 μL;反应条件:42 ℃ 15 min,95 ℃ 3 min。参照荧光定试剂盒说明书检测。
-
收集各组A549细胞(2.5×105个/毫升)接种于96孔板(100微升/孔),每孔加入MTT溶液20 μL,于培养箱内继续培养4 h后弃上清液,每孔加入150 μL DMSO后室温孵育5 min,应用多功能酶标仪检测各孔吸光度(OD)值并计算细胞存活率[实验组OD值/对照组OD值×100%]。
-
收集各组A549细胞接种于6孔板,每孔加入细胞数量为500个,于培养箱内继续培养14 d,每3 d更换一次培养液,培养结束后弃培养液,加入甲醇500 μL,将其置于-20 ℃冰箱内孵育20 min,弃甲醇后加入1%结晶紫染色液400 μL,染色时间为15 min,采用蒸馏水洗涤后晾干。
-
收集各组A549细胞加入预冷PBS洗涤,弃上清后收集细胞沉淀,将500 μL结合缓冲液加入其中后分别加入5 μL Annexin V-FITC、5 μL PI,室温振荡孵育10 min后应用FACS Calibur流式细胞仪检测细胞凋亡率。
-
取对数生长期A549细胞(2.5×105个/毫升)接种于6孔板(100微升/孔),在培养板底部水平画线,继续培养48 h后测量细胞迁移距离。
-
LncBase v.2预测显示LRRC75A-AS1与miR-22-3p存在结合位点,构建野生型载体WT-LRRC75A-AS1、突变型载体MUT-LRRC75A-AS1,miR-NC、miR-22-3p mimics分别与WT-LRRC75A-AS1、MUT-LRRC75A-AS1共转染入A549细胞后继续培养48 h,收集细胞后检测荧光素酶活性。
-
提取各组A549细胞总蛋白,并检测蛋白浓度,检测方法参照BCA蛋白定量检测试剂盒说明书,蛋白变性后进行SDS-PAGE,转膜后使用5%脱脂奶粉封闭2 h,加入一抗稀释液(1∶1 000)(美国Santa Cruz)后置于4 ℃冰箱内孵育24 h,加入二抗稀释液(1∶2 000)(武汉艾美捷),将其置于室温条件下孵育1 h,滴加ECL,应用ImageJ软件分析各条带灰度值。
-
采用t检验和单因素方差分析。
1.1. 材料与试剂
1.2. 方法
1.2.1. 实验分组
1.2.2. qRT-PCR检测LRRC75A-AS1、miR-22-3p的表达水平
1.2.3. MTT检测细胞增殖
1.2.4. 平板克隆形成实验
1.2.5. 流式细胞术检测细胞凋亡率
1.2.6. 划痕实验检测细胞迁移能力
1.2.7. 双荧光素酶报告基因检测LRRC75A-AS1与miR-22-3p的靶向关系
1.2.8. Western blotting检测Cleaved-caspase3蛋白表达
1.3. 统计学方法
-
与癌旁组织比较,肺癌组织中LRRC75A-AS1的表达水平升高(P < 0.01),miR-22-3p的表达水平降低(P < 0.01)(见表 1)。
分组 n LRRC75A-AS1 miR-22-3p 癌旁组织 43 1.01±0.13 1.00±0.12 肺癌组织 43 4.59±0.34 0.28±0.04 t — 64.49 37.33 P — < 0.01 < 0.01 -
与si-NC组比较,si-LRRC75A-AS1组细胞存活率降低(P < 0.01),克隆形成数减少(P < 0.01),凋亡率升高(P < 0.01),Cleaved-caspase3蛋白水平升高(P < 0.01)(见表 2)。
分组 存活率/% 克隆形成数/个 凋亡率/% Cleaved-caspase3 si-NC 100.00 121.00±5.10 8.42±0.34 0.20±0.01 si-LRRC75A-AS1 54.45±2.21 58.67±2.49 25.59±0.80 0.68±0.05 t 35.70 19.02 34.21 16.31 P < 0.01 < 0.01 < 0.01 < 0.01 -
与si-NC组比较,si-LRRC75A-AS1组划痕愈合率降低(P < 0.01),miR-22-3p的表达水平升高(P < 0.01)(见表 3)。
分组 LRRC75A-AS1 miR-22-3p 划痕愈合率/% si-NC 0.99±0.05 0.99±0.04 66.71±1.43 si-LRRC75A-AS1 0.23±0.01 3.47±0.07 32.56±0.95 t 25.82 53.28 34.45 P < 0.01 < 0.01 < 0.01 -
LncBase v.2预测显示LRRC75A-AS1与miR-22-3p存在结合位点(见图 1)。miR-22-3p过表达可降低野生型载体WT-LRRC75A-AS1的荧光素酶活性(P < 0.01),而对突变型载体MUT-LRRC75A-AS1的荧光素酶活性无明显影响(P>0.05)(见表 4)。
分组 WT-LRRC75A-AS1 MUT-LRRC75A-AS1 miR-NC 0.98±0.05 1.00±0.05 miR-22-3p 0.24±0.01 0.99±0.03 t 25.14 0.30 P < 0.01 >0.05 -
与si-LRRC75A-AS1+anti-miR-NC组比较,si-LRRC75A-AS1+miR-22-3p inhibitor组细胞存活率升高(P < 0.01),克隆形成数增多(P < 0.01),凋亡率降低(P < 0.05),划痕愈合率升高(P < 0.01),Cleaved-caspase3蛋白水平降低(P < 0.01)(见表 5)。
分组 存活率/% 克隆形成数/个 凋亡率/% 划痕愈合率/% Cleaved-caspase3 si-LRRC75A-AS1+anti-miR-NC 54.61±2.28 57.67±2.49 25.63±0.99 32.52±0.98 0.67±0.06 si-LRRC75A-AS1+miR-22-3p inhibitor 88.75±3.22 106.67±3.68 13.11±0.55 55.30±1.18 0.30±0.03 t 14.99 19.10 19.15 25.72 9.55 P < 0.01 < 0.01 < 0.01 < 0.01 < 0.01
2.1. LRRC75A-AS1和miR-22-3p在肺癌中的表达
2.2. 干扰LRRC75A-AS1对A549细胞增殖、凋亡的影响
2.3. 干扰LRRC75A-AS1对A549迁移的影响
2.4. LRRC75A-AS1靶向miR-22-3p
2.5. 抑制miR-22-3p可逆转干扰LRRC75A-AS1对A549增殖、凋亡、迁移的影响
-
LncRNA-miRNA-mRNA分子轴在肺癌发生及发展过程中的作用机制尚未完全阐明,LncRNA MALAT1通过调节miR-200a-3p促进非小细胞肺癌的进展[7]。LncRNA FEZF1-AS1通过调节WNT途径而增强非小细胞肺癌细胞上皮-间质转化(EMT)[8]。LncRNA linc00673通过充当miR-150-5p的海绵分子而调节非小细胞肺癌细胞增殖、迁移、侵袭及EMT[9]。LncRNA LINC00342通过靶向miR-203a-3p调节非小细胞肺癌细胞生长和转移[10]。但仍有部分LncRNA在肺癌中的作用机制尚未阐明。
LRRC75A-AS1在结直肠癌中表达水平降低,但相关研究[11-12]表明LRRC75A-AS1在急性髓细胞性白血病中表达水平升高。本研究结果显示,肺癌组织中LRRC75A-AS1的表达水平升高,干扰LRRC75A-AS1表达可明显降低肺癌细胞存活率,还可减少克隆形成数,提示干扰LRRC75A-AS1表达可抑制肺癌细胞增殖及克隆形成能力。caspase3激活后形成Cleaved-caspase3从而诱导细胞凋亡[13-14]。本研究结果显示,干扰LRRC75A-AS1表达可提高肺癌细胞凋亡率及Cleaved-caspase3的表达水平,提示干扰LRRC75A-AS1表达可促进肺癌细胞凋亡。同时本研究结果显示,干扰LRRC75A-AS1表达可明显降低划痕愈合率,提示干扰LRRC75A-AS1表达可抑制肺癌细胞迁移。
本研究证实LRRC75A-AS1可充当miR-22-3p的海绵分子,并可负向调控miR-22-3p的表达。LncRNA LINC00858通过充当miR-22-3p的竞争性内源RNA而促进结肠直肠癌细胞增殖、迁移和侵袭[15]。LncRNA DGCR5通过抑制miR-22-3p促进肺腺癌发展进程[16]。LncRNA NCK1-AS1通过miR-22-3p/IGF1R途径增强神经胶质瘤细胞的增殖[17]。LncRNA H19通过miR-22-3p/Snail1轴调控胃癌的细胞生长和转移[18]。本研究结果显示,肺癌组织中miR-22-3p的表达水平降低,抑制miR-22-3p可逆转干扰LRRC75A-AS1对肺癌细胞增殖、凋亡及迁移的作用。
综上所述,肺癌组织中LRRC75A-AS1的表达水平升高,而miR-22-3p的表达水平降低,干扰LRRC75A-AS1表达可通过上调miR-22-3p的表达从而抑制肺癌细胞增殖、迁移及克隆形成能力,并可促进肺癌细胞凋亡。但LRRC75A-AS1是否可通过调控miR-22-3p及其靶基因表达从而发挥抗肺癌作用仍需进一步探究。







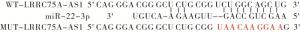
 DownLoad:
DownLoad: