-
原发性肝癌是一种高度恶性肿瘤,在引起肿瘤相关性死因中居第二位,大多数肝癌病人就诊时已处于进展阶段,无法接受根治性治疗,预后差,复发率和病死率高[1-2]。肝细胞癌(hepatocellular carcinoma, HCC)约占原发性肝癌中的90%[2]。欧洲肝脏病学会和美国肝脏病学会推荐的治疗HCC的主要治疗手段包括手术切除、经动脉化疗栓塞术、肝移植及局部消融等[3]。尽管近年来这些手段有了一定的发展,但由于肝癌转移和复发率较高,预后仍较差。所以,迫切需要寻找新的治疗方法。
近年来,伴随着免疫学、分子生物学、生物工程的发展,肿瘤免疫治疗已成为最有潜力的治疗手段之一。肿瘤中浸润的巨噬细胞称肿瘤相关巨噬细胞(tumor associated macrophages,TAM),为一类重要的免疫细胞。TAM被认为主要是M2型巨噬细胞[4],主要作用是免疫抑制,促进血管生成,分泌多种促进肿瘤生长的因子等。核因子κB(nuclear factor κB,NF-κB)是一种细胞核转录因子,广泛存在于真核细胞中。NF-κB参与了机体炎症反应、免疫应答及细胞凋亡等,可以通过调节细胞因子等,促进肿瘤血管生成、浸润和转移。NF-κB p50是其重要家庭成员之一,对于NF-κB蛋白在肿瘤中扮演的角色起到重要作用。但NF-κB与M2巨噬细胞的关系尚不明确。本文研究了肝癌中M2巨噬细胞及NF-κB p50的表达情况,探讨它们在不同分期肝癌中表达的相关性。
HTML
-
本研究整群收集2012-2015年在安徽医科大学第一附属医院初次诊断并接受手术的80例HCC病人和10例肝内胆管结石病人(对照组)的石蜡标本;2015年10月至2016年1月在安徽医科大学第一附属医院肝胆外科手术切除的HCC病人肝癌新鲜组织20例及癌旁新鲜组织6例(对照组)。取分界线以外相应一侧2 cm的组织为癌旁组织。全组病例需符合的条件有:(1)手术前未接受过化疗或放疗;(2)有TNM分期等资料;(3)标本组织学经安徽医科大学第一附属医院病理科专家再次确诊为HCC。
HCC病人肝组织石蜡切片中临床分期Ⅰ+Ⅱ期病人34例(42.5%),Ⅲ+Ⅳ期46例(57.5%);HCC病人新鲜肝组织标本中临床分期Ⅰ+Ⅱ期病人12例(60%),Ⅲ+Ⅳ期病人8例(40%)。分期标准按照美国肿瘤联合会(American Joint Committee on Cancer, AJCC)2010年对原发性肝癌分期(第六版)制定的TNM分期。
-
CD163、CD206抗体(美国Santa Cruz公司);AlexaFluor594、AlexaFluor488荧光二抗(美国ThermoFisher Scitific公司);无水乙醇、甲醇、二甲苯(上海中试化总公司);Triton X-100和DAPI(上海碧云天生物技术公司);山羊血清(美国Gibco公司);分离胶缓冲液、浓缩胶缓冲液、Western一抗、二抗稀释液(江苏碧云天公司);BCA蛋白定量试剂盒(美国Thermo)。
石蜡切片机、激光共聚焦显微镜[德国莱卡(Leica)仪器有限公司];电泳仪(美国BIO-RAD公司);4 ℃冰箱(海尔);高速台式冷冻离心机(美国Sigma公司)。
-
HCC组织经甲醛固定,梯度乙醇脱水后,将组织和蜡块置于包埋框内,冷却后即成蜡块。进行防脱处理后,Leica切片机切成厚4~6 μm薄片,将蜡片裱贴于载玻片上,烤干待用。梯度乙醇脱蜡后,用Triton浸润切片。用枸橼酸盐修复抗原后,滴加3%过氧化氢阻断内源性过氧化酶。玻片上滴加山羊血清室温下孵育20 min,PBS洗3次,每次3 min。CD163和CD206均以1: 50稀释,混合后滴加在切片组织上,放置于湿盒中,4 ℃孵育过夜后,将湿盒从冰箱取出,复温,PBS洗3次,每次3 min。在切片组织上滴加羊抗兔或羊抗鼠(稀释度1: 100)荧光二抗,37 ℃避光孵1 h;PBS洗3次,每次3 min。在切片组织上滴加DAPI染色,室温避光孵育10 min,PBS洗3次,每次3 min。滴加抗荧光淬灭剂后,将盖玻片覆盖在组织上,固定后,显微镜下观察并拍照。
-
将RIPA和PMSF按100: 1配制裂解液。从-80 ℃冰箱取出冻存肝脏组织,每例剪取约50 mg,用PBS冲洗,放入匀浆器中,按10 μL/mg组织加入裂解液。冰上研磨10 min,4 ℃、14 000 r/min离心20 min,吸取上清,即为总蛋白。
-
配制10%的SDS-聚丙烯酰胺分离胶, 进行上样、电泳、转膜等操作,用5%脱脂奶粉室温下封闭2 h。然后加入一抗4 ℃孵育过夜。TBST洗3次,每次5 min。加入二抗室温孵育2 h,再用TBST洗3次,每次5 min后,ECL试剂盒显影,Image-J软件分析图片。以β-actin为内参,结果以目的条带与β-actin条带灰度值的比值作为目的蛋白的相对表达量。
-
采用方差分析、q检验和直线相关分析。
1.1. 研究对象
1.2. 试剂与仪器
1.3. 方法
1.3.1. 免疫荧光染色检测CD163和CD206的共表达
1.3.2. 新鲜肝癌组织蛋白的提取
1.3.3. Western blotting检测CD163、CD206及NF-κB p50的表达
1.4. 统计学方法
-
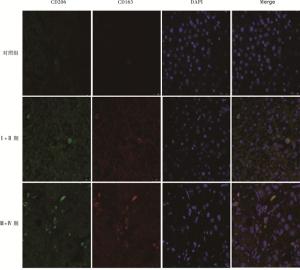
绿色荧光标记CD206蛋白,红色荧光标记CD163蛋白,DAPI染细胞核,Merge可显示绿色和红色荧光共定位情况,两种荧光重合的细胞表示该细胞存在CD206和CD163的共定位。免疫荧光双染结果显示,对照组中CD163和CD206的共表达较少,且荧光强度较弱,而TNMⅠ+Ⅱ期病人肝组织切片中CD163和CD206的共表达增多,荧光强度增加。随着肝癌进展,TNM Ⅲ+Ⅳ期中CD163和CD206的共表达明显比TNMⅠ+Ⅱ期病人增多,且荧光强度明显增强(见图 1)。
-
Western blotting结果显示,CD163和CD206在肝癌病人肝组织中表达上升, Ⅲ+Ⅳ期病人上升较Ⅰ+Ⅱ期病人更为明显(P<0.05~P<0.01)(见图 2、表 1)。
分组 CD163 CD206 p50 对照组 0.32±0.05 0.34±0.05 0.31±0.04 Ⅰ+Ⅱ期 0.48±0.07** 0.62±0.04* 0.85±0.04** Ⅲ+Ⅳ期 0.81±0.08**△△ 0.88±0.07**△△ 1.79±0.04**△△ F 40.044 66.482 991.034 P <0.01 <0.01 <0.01 MS组内 0.005 0.003 0.003 注:相对灰度值=目的蛋白灰度值/β-actin灰度值; q检验:与control组比较*P<0.05, **P<0.01;与Ⅰ+Ⅱ期组比较△△P<0.01 -
Western blotting结果显示,NF-κB p50的表达在肝癌病人肝组织中上升, Ⅲ+Ⅳ期病人上升较Ⅰ+Ⅱ期病人更为明显(P<0.01)(见图 3、表 1)。
-
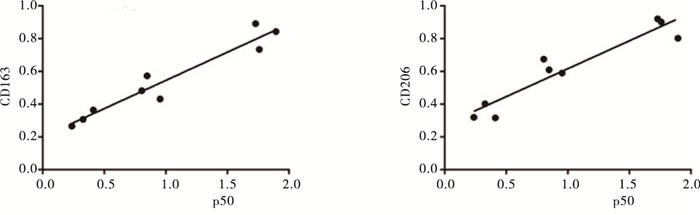
相关性分析发现,肝癌病人肝脏组织中NF-κB p50与CD163、CD206的表达在各组中呈现明显正相关(r=0.963, P<0.01;r=0.944, P<0.01)(见图 4)。
2.1. HCC病人肝组织切片中CD163和CD206的共表达
2.2. HCC病人新鲜肝组织中CD163和CD206的表达
2.3. HCC病人肝脏组织中NF-κB p50的表达
2.4. HCC病人肝脏组织中M2巨噬细胞与NF-κB p50表达的相关性
-
HCC为消化系统常见肿瘤之一,病因尚不完全明确,且缺乏有效的治疗手段[5-7]。HCC是多步骤、多阶段发生的疾病,高侵袭性和转移能力是其特点,也是影响病人疗效和预后的主要因素。HCC的发生、发展受到多种因素的影响。肿瘤微环境已被证实在肿瘤的发展转移和复发过程中起到重要作用,肿瘤微环境包括肿瘤细胞、细胞外基质和基质细胞(包括成纤维细胞、免疫和炎性细胞、脂肪细胞等)[8],而巨噬细胞是肿瘤微环境中浸润的一类重要免疫细胞。
在本研究中,CD163和CD206被用来标记M2巨噬细胞,检测M2巨噬细胞在不同分期肝癌样本中的表达。CD163是一种在人体中被CD163基因编码的蛋白质,是对血红蛋白-结合珠蛋白复合物高亲和力的清道夫受体, 其是一类吞噬细胞表面的跨膜蛋白。CD163仅仅在单核-巨噬细胞系中表达,在多种含成熟巨噬细胞的组织器官中高表达,如肺泡巨噬细胞、脾脏的血浆巨噬细胞、肝脏巨噬细胞(枯否细胞)等[9]。CD206,即甘露糖受体1,是一种主要存在于巨噬细胞和未成熟DC细胞的C型凝集素。有报道[10]称,其可促进巨噬细胞活化、抗原提呈和免疫应答。CD163和CD206是公认的鉴定M2型巨噬细胞的标志物[11-12],研究它们的共定位更能准确地定位M2型巨噬细胞。在本研究中,CD163和CD206在肝癌组织石蜡切片中的共定位明显高于对照组表达,同时在Ⅲ+Ⅳ期的共定位明显高于Ⅰ+Ⅱ期,提示M2巨噬细胞的表达可能与HCC的恶性度及转移有关。肝癌新鲜组织的Western blotting结果也显示,M2巨噬细胞在肝癌组织中的表达高于癌旁组织,同时晚期肝癌中CD163和CD206的表达明显高于早期肝癌。
NF-κB家庭主要成员包括RelA(p65)、c-Rel和RelB、NF-κB1(p50蛋白及其前体p105)、NF-κB2(p52蛋白及其前体p100)。有研究[13]表明,NF-κB可通过持续活化促进肿瘤细胞增殖,减少凋亡,提高肿瘤细胞的浸润和转移能力[13]。在哺乳动物中,p50是最重要的蛋白之一,参与了多种肿瘤细胞的表达,发挥重要作用。经典的NF-κB信号通路参与了机体的自身免疫[14],而脊椎动物的炎症反应是自身免疫的重要表现形式。肝癌是典型的炎症-肿瘤相关疾病。本研究结果显示,随着肝癌进展,p50表达明显增高。相似的,PORTA等[15]报道p50参与了结直肠癌的进展。同时M2巨噬细胞的数量也增多,而进一步研究NF-κB p50的表达与M2巨噬细胞的相关性发现,NF-κB p50蛋白的表达与M2巨噬细胞的表达呈显著正相关。既往研究[16-17]表明,TAM可释放多种细胞因子、趋化因子、生长因子、基质金属蛋白酶等,促进肿瘤进展。TAM促进肿瘤生长浸润可能与其高分泌白细胞介素-10、肿瘤坏死因子-α等细胞因子,激活NF-κB通路,上调NF-κB p50蛋白的表达相关。而NF-κB从细胞质进入细胞核内,调控炎性细胞因子的表达,上调信号转导和转录激活因子-3等的表达,进一步促进肿瘤进展[18]。同时,NK-κB p50能诱导M2巨噬细胞相关基因,是M2巨噬细胞驱动的炎症反应中的重要组分[19],提示M2巨噬细胞和NK-κB p50可能存在协同作用,共同促进肝癌进展。
综上所述,随着肝癌的进展,M2巨噬细胞和NK-κB p50的表达均上升,且具有明显相关性,提示M2巨噬细胞增多,可能通过激活NF-κB信号通路,上调p50表达,促进了HCC的恶性进程;而p50表达的上升可能进一步增多M2巨噬细胞,从而进一步促进肝癌的进展。本研究结果有助于进一步了解M2型巨噬细胞促进肿瘤浸润生长的机制,为肝癌靶向治疗提供新线索。










 DownLoad:
DownLoad: