-
随着人口老龄化进程加快,骨质疏松症(osteoporosis, OP)和2型糖尿病病人(type 2 diabetes mellitus,T2DM)发病率逐年升高。在糖尿病的发生发展过程中,也伴随骨代谢的异常[1]。糖尿病所引发的骨质疏松可能与T2DM病人处于高血糖状态、体内胰岛素的缺乏、脂质代谢紊乱及炎症因子所引起的破骨细胞活动增强等有关[2]。已有研究[3]发现,胸腰椎交界处因椎体活动度大,是骨折的好发部位,其中,胸腰段椎体常指T11~L2。糖尿病所引起的椎体骨折,会引发严重的并发症,其致死、致残率较高[4]。因此,本研究通过定量CT(quantitative CT,QCT)测量胸T11~L4椎体的骨密度(bone mineral density,BMD),探讨T2DM病人胸腰椎BMD与正常人群胸腰椎BMD的差异及OP检出率,为糖尿病性OP提供更好的防治依据。
HTML
-
选取2021年3-12月在江苏省中医院住院治疗的T2DM病人68例作为观察组。排除标准: (1)排除1型、2型及特殊类型糖尿病;(2)临床资料不全且未做QCT BMD检查的病人;(3)其余同T2DM病人的排除标准。纳入标准: (1)符合2020年美国糖尿病学会诊断为T2DM的标准(或已经确诊为T2DM病人);(2)进行常规的实验室检查[空腹血糖(fasting blood glucose,FBG)或糖化血红蛋白]且临床基线资料完整;(3)所有T2DM病人均进行QCT BMD检查。排除标准: (1)排除严重肾功能障碍,合并糖尿病急性并发症;(2)恶性肿瘤、甲状旁腺疾病、垂体疾病等影响骨代谢的病人;(3)骨关节疾病、椎体压缩性骨折、椎体有病变(如: 椎管淋巴瘤、转移瘤、椎体血管瘤等疾病)等影响BMD测量的病人;(4)心功能不全、心绞痛、心肌梗死等疾病;(5)合并风湿性疾病,或内分泌系统其他疾病,如结缔组织病、系统性红斑狼疮、性腺功能异常等疾病;(6)曾经服用过影响骨代谢的药物,如皮质类固醇激素、雌激素、钙剂、维生素D等;(7)年龄 < 20岁或>70岁的病人。糖尿病诊断标准: 采用2020年美国糖尿病学会《糖尿病医学诊疗标准》, FBG≥7 mmol/L和(或)之前确诊为T2DM,正在服用降血糖药物[5]。OP诊断标准[6]: BMD>120 mg/cm3为正常;BMD 80~120 mg/cm3为低骨量;BMD < 80 mg/cm3为OP。选取年龄和性别相匹配的同期健康体检者96名作为对照组。
2组再按照体质量指数(bone mass index,BMI)分3个亚组: BMI < 24 kg/m2组、BMI 24~ < 28 kg/m2组、BMI≥28 kg/m2组;按年龄分4个亚组: < 40岁组、40~ < 50岁组、50~ < 60岁组、60~70岁组;按性别分为男性组和女性组。
-
所有受试者进行全腹部CT平扫,病人取仰卧位,头先进,扫描范围从膈顶至耻骨联合。采用美国GE 64排Optima CT660扫描机及美国Mindways公司的QCT Pro 4BMD软件进行检查和分析;扫描参数: 电压120 kV,管电流349 mA,FOV 500 mm,将全腹部CT扫描数据传至Mindways公司的QCTPro 4BMD分析工作站,采用“New 3D spine exam analysis”功能进行T11~L4椎体BMD测量,计算平均BMD。
-
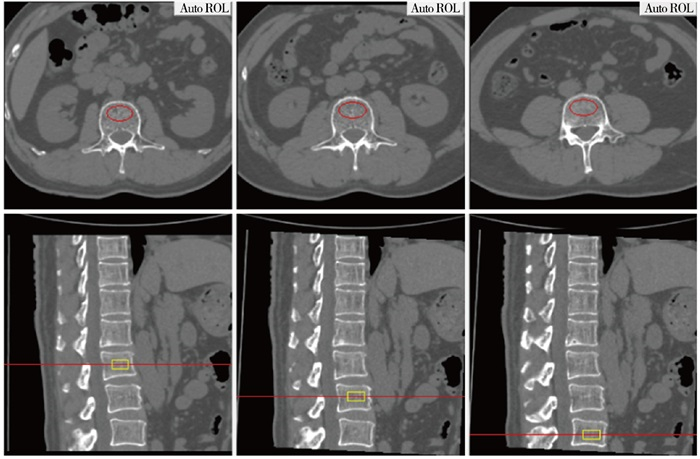
图像传至QCT Pro工作站后,使用“New 3D spine exam analysis”功能软件,在横断位的定位图像上,将红色定位感兴趣区(region of interest,ROI)放在所测量椎体的中心,同时在矢状位定位图像上,黄色矩形ROI自动定位所测量的椎体上下边缘。放置ROI应注意: 放置在所测量椎体的中心;ROI层厚选择9 mm;ROI放置距皮质骨边缘2~3 mm;ROI尽可能多地包括骨松质,应避开增生骨及椎体后部的中央静脉(见图 1)。所测量的BMD值自动输出后处理软件。
-
比较2组一般资料、不同年龄分组胸腰椎(T11~L2)OP发生率、不同BMI分组胸腰椎BMD和不同性别分组胸腰椎BMD。
-
采用t检验、χ2检验、秩和检验和Fisher′s确切概率法。
1.1. 一般资料
1.2. 方法
1.3. 数据测量
1.4. 观察指标
1.5. 统计学方法
-
2组受试者性别、年龄差异均无统计学意义(P>0.05);观察组病人BMI和FPG均高于对照组(P < 0.05和P < 0.01);观察组从T11~L4椎体的BMD均低于对照组(P < 0.05~P < 0.01)(见表 1)。
分组 n 男 女 年龄/岁 BMI/(kg/m2) FPG/(mmol/L) T11 BMD/(mg/cm3) T12 BMD/(mg/cm3) L1 BMD/(mg/cm3) L2 BMD/(mg/cm3) L3 BMD/(mg/cm3) L4 BMD/(mg/cm3) 观察组 68 41 27 52.0±10.5 25.0±3.6 6.6±2.4 132.7±38.0 128.7±33.5 126.2±33.0 122.9±34.5 118.2±34.3 120.2±35.6 对照组 96 45 51 49.4±11.8 23.7±3.0 4.8±0.5 149.2±35.4 144.1±36.0 141.5±36.2 138.7±38.4 133.2±36.8 133.4±37.3 t — 2.87△ 1.45 2.51 5.96* 2.85 2.78 2.76 2.71 2.64 2.28 P — >0.05 >0.05 < 0.05 < 0.01 < 0.01 < 0.01 < 0.01 < 0.01 < 0.01 < 0.05 △示χ2值;*示uc值 -
随着年龄增加,2组T11~L2椎体发生OP的概率增加(P < 0.01)(见表 2)。
分组 观察组(n=68) 对照组(n=96) 正常骨量 骨量减少 OP 正常骨量 骨量减少 OP < 40岁 7(100.0) 0(0) 0(0) 20(100.0) 0(0) 0(0) 40~ < 50岁 16(80.0) 4(20.0) 0(0) 24(92.3) 2(7.7) 0(0) 50~ < 60岁 11(42.3) 14(53.9) 1(3.8) 20(58.8) 14(41.2) 0(0) 60~70岁 5(33.3) 6(40.0) 4(26.7) 4(25.0) 8(50.0) 4(25.0) P < 0.01△ < 0.01△ △示Fisher′s确切概率法 -
当BMI < 24 kg/m2,观察组胸腰椎BMD均低于对照组(P < 0.05);当BMI 24~ < 28 kg/m2和BMI≥28 kg/m2时,2组胸腰椎BMD差异均无统计学意义(P>0.05)(见表 3)。
分组 n T11~T12 L1~L4 BMI < 24 kg/m2 观察组 29 124.5±37.7 115.9±35.1 对照组 52 155.7±35.0 145.1±37.1 t — 3.74 3.38 P — < 0.01 < 0.01 BMI 24~ < 28 kg/m2 观察组 26 132.4±32.7 123.5±29.4 对照组 36 134.7±34.7 123.9±35.1 t — 0.26 0.05 P — >0.05 >0.05 BMI≥28 kg/m2 观察组 13 141.5±34.4 131.9±29.6 对照组 8 141.7±25.3 138.3±27.2 t — 0.02 0.50 P — >0.05 >0.05 -
在女性组,观察组胸腰椎BMD均低于对照组(P < 0.05~P < 0.01);在男性组,2组胸腰椎BMD差异均无统计学意义(P>0.05)(见表 4)。
分组 n T11 BMD T12 BMD L1 BMD L2 BMD L3 BMD L4 BMD 男性 观察组 41 137.4±36.0 132.3±32.0 129.2±32.5 126.8±32.5 131.0(97.2, 147.8) 125.7±33.2 对照组 45 144.1±30.0 138.9±31.9 137.0±30.1 134.8±32.6 131.1±30.9 132.0±32.6 t — 0.95 0.96 0.86* 1.13 0.75* 0.79* P — >0.05 >0.05 >0.05 >0.05 >0.05 >0.05 女性 观察组 27 125.8±40.6 123.2±35.7 121.5±33.7 117.0±37.1 109.5±35.7 111.8±38.1 对照组 51 153.7±39.3 148.6±39.1 145.6±40.2 142.2±42.9 134.9±41.6 134.6±41.3 t — 2.96 2.80 2.65 2.59 2.69 2.38 P — < 0.01 < 0.01 < 0.01 < 0.05 < 0.01 < 0.05 *示uc值
2.1. 2组一般资料比较
2.2. 2组不同年龄分组胸腰椎OP发生率比较
2.3. 2组不同BMI分组胸腰椎BMD比较
2.4. 2组不同性别分组胸腰椎BMD比较
-
脊椎主要是由颈椎、胸椎、腰椎及骶尾骨等组成,胸腰椎交界处是脊椎椎体活动度最大的部位,该部位是胸椎后突与腰椎前突的交接点,也是压缩性骨折的好发部位。目前研究[7]已证实1型糖尿病会引起椎体BMD的下降,较正常人更易发生OP,即1型糖尿病会引起骨代谢的紊乱,OP的发生率增加。然而,T2DM是否会引起椎体BMD发生改变还存在争议。ABDULAMEER等[8]对T2DM病人与BMD之间的相关性47篇文献进行分析,发现有的文献表明T2DM病人的BMD是增高的,有的T2DM病人BMD是降低的,还有的报道T2DM病人与对照组之间的BMD并不存在差异。本研究发现随着年龄的增加,2组胸腰椎BMD均呈逐渐降低的趋势,且T2DM病人的BMD均低于正常人群。
糖尿病所引起的胸腰椎压缩性骨折是OP常见的骨折类型,OP是一种骨代谢异常导致的骨骼疾病,主要以骨强度和BMD下降为特征,增加了骨折风险[9]。在国际上,OP的诊断和治疗主要依赖BMD测量和骨代谢指标的变化,临床上多是通过检测BMD的变化评估有无OP。目前双能X线吸收法(dual-energy X-rays absorptiometry,DXA)是认可度最高的BMD测量方法,但DXA测量是面积BMD,对于骨质增生、血管钙化及骨折的人群会导致测量结果不准确[6]。而QCT通过测量体积BMD将骨皮质和骨松质分开,不易受到骨质退变、血管钙化等影响,对骨量细微改变较敏感,测量结果更加精准,对早期预测OP更加精准和敏感。本研究发现,随着年龄的增加,胸腰椎交界处发生OP的概率也增加,且观察组发生OP的概率高于对照组。胸腰椎交界处是骨质疏松性骨折的好发部位可能与胸、腰椎椎体的结构和位置有关,胸椎椎体骨松质含量较腰椎椎体少,胸椎的稳定性高于腰椎等其他区域且胸椎较腰椎承重轻[10]。
目前,BMD与BMI之间的关系存在争议,LAU等[11]对亚洲男性BMD与BMI之间的研究发现,BMI与BMD呈正相关关系,BMI是BMD的保护因素。但是,也有研究[12]表明肥胖与BMD呈负相关关系,推测其原因可能是脂肪细胞和成骨细胞都来源于间质干细胞,两者存在抵抗关系,所以脂肪细胞会影响骨代谢。本研究结果发现,在BMI < 24 kg/m2组,观察组胸腰椎BMD均低于对照组;在其他BMI分组中,2组之间的胸腰椎BMD差异无统计学意义,说明BMI可能是BMD的保护因素,其机制可能是机械负荷增加刺激成骨细胞增生,从而促进椎体BMD增加,与ZHOU等[13]研究结果一致。性别也是影响骨质疏松性骨折的重要因素,本研究发现在女性组中,观察组病人胸腰椎BMD均低于对照组,与之前的研究[14]结果一致。出现这种现象的原因可能与T2DM女性的BMD降低受激素的影响,特别是雌激素,若骨骼中雌激素受体减少,会造成骨吸收和骨重建发生失调,骨量丢失加快,造成骨强度的下降,导致OP发病率的增加[15]。
综上,T2DM病人椎体BMD降低和性别有密切的联系,特别是女性T2DM病人为OP发生的高危人群,提示女性T2DM病人应加强BMD的相关检查,重视骨骼的全面评估;随着年龄的增加,胸腰椎BMD均呈下降趋势,T2DM病人发生OP的概率更高,提示T2DM病人不仅要重视糖尿病本身的治疗,也应该应重视BMD的检查,因为OP带来的骨折所造成的严重后果往往会超过糖尿病本身。同时本研究也有局限性,如纳入的样本量较少,单中心研究,可能会影响最终的研究结果。在后续研究中,会继续增大样本量进行多中心的研究。







 DownLoad:
DownLoad: