-
系统性红斑狼疮(systemic lupus erythematosus,SLE)是一种全身性自身免疫性疾病,以自身免疫失衡、产生多种自身抗体为特征,通过形成免疫复合物,激活多种炎症反应,进而导致多器官损伤[1-2]。树突状细胞(dendritic cells, DCs)作为重要的抗原递呈细胞(antigen-presenting cell, APCs)之一,被认为在SLE疾病的起始、进展和延续中发挥关键作用,DCs依据功能来源不同可分为不同的DC亚群,包括髓样树突状细胞(mDC)和浆细胞样DC (plasmacytoid dendritic cells,pDC)[3-4]。Toll样受体(Toll-like receptors,TLRs)是模式识别受体的主要类型之一,在天然免疫中起重要作用。越来越多的证据表明,TLRs通过病原体相关分子模式(Pathogen-associated molecular patterns, PAMPs)识别自身分子,参与了SLE等自身免疫性疾病的发病过程。而以往较多研究[5-7]关注于细胞内TLRs如TLR7、TLR9等的表达对SLE发病的影响, 对于TLR2、TLR4的报道相对较少,特别是对于髓系来源的专职抗原递呈细胞mDC上TLR2、TLR4的表达水平与SLE病情的关系尚乏研究,故本实验旨在通过检测mDC膜表面TLR2、TLR4的表达情况及血清白细胞介素(IL)-10、IL-17A表达水平进一步探讨其在SLE发病机制中的作用。
HTML
-
选取2021年3月至2022年1月于蚌埠医科大学第一附属医院风湿免疫科治疗的54例SLE病人作为研究对象,纳入标准:(1)符合1997年美国风湿病学会制定的SLE分类标准;(2)除外其他结缔组织病以及肿瘤、结核等传染病。54例SLE病人中男7例,女47例;年龄15~74岁,平均(42.15±13.93)岁。根据SLEDAI-2000评分标准[8]将其分为稳定组(≤4分)21例和活动组(≥5分)33例。同时选取本院30名健康志愿者为对照组,其中男8例,女22例,年龄22~69岁,平均(38.73±15.51)岁。SLE病人组与对照组在年龄和性别上差异无统计学意义(P>0.05), 具有可比性。所有研究对象均签署知情同意书。
-
流式抗体包括Lin1(CD3/14/16/19/20/56)-FITC、HLA-DR-APC-CY7、CD11c-APC、TLR4-PE、IgG2a-PE、TLR2-PE-Cy7和IgG2a-PE-Cy7均购自美国BioLegend公司;红细胞裂解液购自Biosharp公司;Human TruStain FcXTM购自美国BioLegend公司;其他试剂如磷酸盐缓冲溶液(PBS)、染色缓冲液(SB)、2%多聚甲醛溶液(PFA)等均为自行配制;IL-10、IL-17A检测试剂盒购自武汉华美生物工程有限公司;流式细胞仪为DxP AthenaTM流式细胞仪,酶标仪为科华KHB-ST-360型。
-
所有研究对象均于清晨空腹状态下采集外周静脉血5 mL, 置于离心机中以4 ℃,1 500 r/min离心5 min后留取上清液并于-80 ℃冰箱中保存备用。然后向剩余血液中加入约6~10倍细胞体积的红细胞裂解液,室温裂解5 min后,再置于离心机中以4 ℃,1 500 r/min离心5 min,弃去上清液(若细胞裂解不完全可重复上述步骤1~2次),加入约5倍体积PBS洗涤,再以上述条件离心后,弃去上清液,加入适当PBS混匀,并移入相应流式管中,向各管细胞悬液中加入5 μL FcR封闭剂,置于4 ℃冰箱中封闭10 min后向各管中加入相应流式抗体,于冰上孵育30 min,向各流式管加入1 mL SB洗涤并离心,弃去上清液,加入200~300 μL 2%PFA固定,而后上机检测。
-
将流式细胞仪电压及补偿调节完毕后,在FSC/SSC散点图上设门,然后通过FSC/FSC-W去除粘连细胞,再以Lin1/HLA-DR设十字门,得到Lin1-HLA-DR+细胞群,在此基础上以CD11c/CD123设十字门得到CD11c+CD123-细胞群即为mDC细胞群,通过同型对照组设置每组TLR2/4细胞群位置。每组样本同型对照阳性率在5%以内。
-
采用ELISA法检测SLE病人和健康志愿者血清中IL-10、IL-17A表达水平,所有实验操作严格按照各试剂盒的说明书进行。
-
采用t检验、单因素方差分析和多重比较(Bonferroni法)、秩和(Mann-Whitney和Kruskal-Walli)检验和Pearson相关性分析。
1.1. 一般资料
1.2. 方法
1.2.1. 主要试剂及仪器
1.2.2. 全血法收集外周血mDC
1.2.3. 流式细胞仪检测外周血mDC表面TLR2、TLR4表达率
1.2.4. ELISA法检测血清中IL-10、IL-17A表达水平
1.3. 统计学方法
-
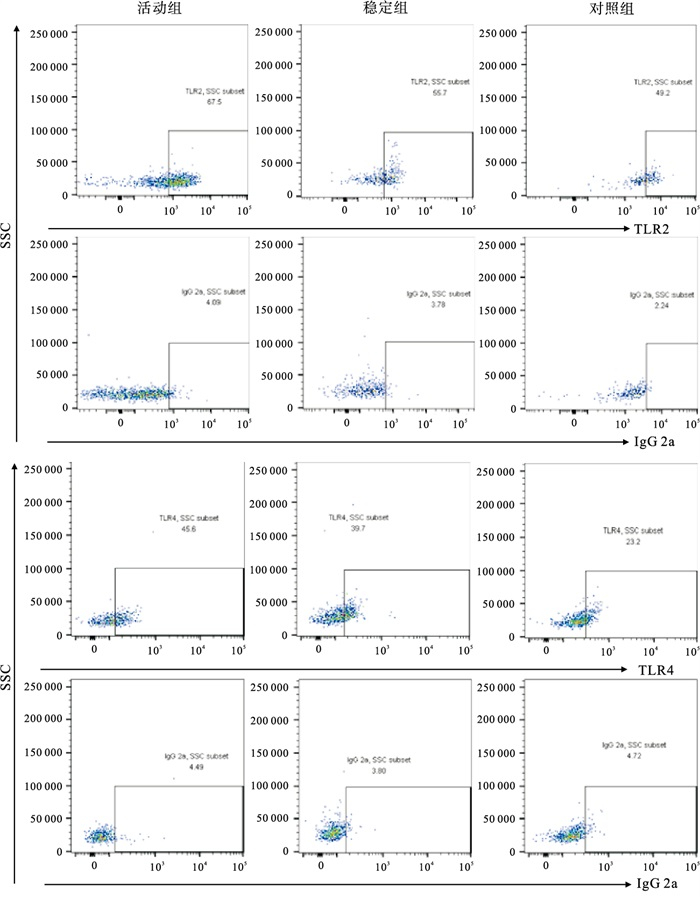
SLE活动组mDC-TLR2的表达率高于稳定组和对照组(P < 0.01);mDC-TLR4的表达率高于对照组(P < 0.01),与稳定组比较差异无统计学意义(P>0.05)(见表 1、图 1)。
分组 n mDC-TLR2表达率
(x±s)/%mDC-TLR4表达率
[M (P25, P75)]/%对照组 30 40.4±17.36 14.35(10.05,31.11) SLE稳定组 21 45.2±20.21 35.75(14.63,51.90) SLE活动组 33 59.4±18.58**△△ 38.87(15.57,64.36)* F — 8.83 10.93# P — < 0.01 < 0.01 MS组内 — 345.210 — 多重比较:与对照组比较*P < 0.05,** P < 0.01;与SLE稳定组比较△△P < 0.01。#示H值 -
SLE活动组和稳定组IL-10、IL-17A表达水平均高于对照组(P < 0.01),活动组与稳定组间差异无统计学意义(P>0.05)(见表 2)。
分组 n IL-10 IL-17A SLE活动组 33 193.59(39.07, 316.75)** 93.35(34.21, 150.38)** SLE稳定组 21 64.236(31.00, 178.27)** 47.58(15.40, 115.35)** 对照组 30 6.58(0.60, 12.57) 4.64(4.36, 5.57) H — 43.7 33.59 P — < 0.01 < 0.01 与对照组比较**P < 0.01 -
在各项实验室指标中,SLE病人外周血mDC-TLR2表达率与血清IgG、24 h尿蛋白定量均呈负相关(r=-0.270、-0.310,P < 0.05),而与抗Sm抗体呈正相关(r=0.340,P < 0.05)。mDC-TLR4表达率与血小板计数呈正相关(r=0.340,P < 0.05),与其余临床指标均无相关性(见表 3)。
实验室指标 mDC-TLR2
阳性率/%mDC-TLR4
阳性率/%白细胞计数/(109/L) 0.177 -0.019 中性粒细胞计数/(109/L) 0.195 -0.057 淋巴细胞计数/(109/L) 0.044 0.107 血红蛋白/(g/L) -0.014 0.181 血小板计数/(109/L) -0.223 0.340* 红细胞沉降率/(mm/h) -0.157 -0.026 C反应蛋白/(mg/L) -0.089 -0.171 IgG/(g/L) -0.270* 0.088 IgA/(g/L) -0.116 0.180 IgM/(g/L) -0.252 0.014 补体C3/(g/L) -0.230 0.083 补体C4/(g/L) -0.111 -0.052 ANA 0.039 -0.011 抗-SSA 0.263 0.109 抗-SSB 0.100 0.043 抗-Sm 0.340* 0.091 抗-RNP 0.105 -0.137 抗dsDNA -0.046 -0.046 抗中性粒细胞胞质抗体 -0.009 0.179 抗CCP抗体 0.013 -0.084 RF 0.238 0.038 ASO -0.010 0.140 25-羟-VD 0.100 -0.129 24 h尿蛋白定量 -0.310* -0.022 *示P < 0.05
2.1. 3组外周血mDC-TLR2、mDC-TLR4表达情况
2.2. SLE病人与对照组血清IL-10、IL-17A表达水平比较
2.3. SLE病人外周血mDC-TLR2、mDC-TLR4表达率与实验室指标相关性
-
SLE是一种慢性自身免疫性疾病,以体内自身抗体产生为特征,可通过形成免疫复合物等激活炎症细胞,促进炎症因子的表达和释放,导致免疫失衡。尽管在过去几十年里对于SLE的早期诊断和治疗有明显进步,但SLE的发病风险和死亡率仍在持续增加[9-12]。因此探索和研究SLE的确切发病机制对于SLE病人的治疗和预后有着十分重要的意义。
TLRs作为重要的模式识别受体,在天然免疫中发挥着重要作用。研究[13-14]报道,TLRs在活化状态下可能通过调节细胞因子的产生而影响SLE疾病活动性,且TLR2和/或TLR4的缺乏使自身抗体的产生减少,延缓了SLE症状的发展。另外,近期一项研究[15]显示使用TLR阻断剂及其衍生物能够显著抑制活化B细胞NF-κB的激活,抑制炎症因子的分泌,并且对SLE小鼠的疾病进展有一定的预防作用,提示TLR2和TLR4在SLE发病中可能起到重要作用。作为较早发现的重要的胞外TLRs,TLR2和TLR4可以识别细菌、支原体、真菌和病毒的各种成分,包括脂蛋白、肽聚糖等,其中TLR2以与TLR1或TLR6形成异源二聚体的形式来识别其配体,而TLR4则通过与细胞膜表面的骨髓分化蛋白2(myeloid differentiation 2,MD2)结合形成复合物而参与配体识别,并招募特定信号分子,包括髓样分化因子88和β-干扰素TIR结构域衔接蛋白,启动下游信号事件,激活转录因子NF-kB,调节炎症细胞因子(如IL-10、IL-17A等)分泌,从而参与免疫反应[16-19]。DCs作为目前已知抗原提呈能力最强的细胞之一,在免疫激活和免疫耐受过程中起到重要作用,依据功能来源不同可分为不同的DC亚群,包括CD11c+CD123- mDC和CD11c-CD123+pDC。mDC可通过提呈抗原、协同信号传递和分泌细胞因子, 激活T细胞, 从而参与免疫反应。人外周血mDC膜表达TLR2、4, 可能通过识别PAMPs,激活前述TLR2/4-MyD88/TRIF通路,释放炎性细胞因子,进而参与SLE的疾病进展[20]。
近几年国内外有关不同细胞膜表面TLR2、TLR4的研究结果不尽相同。有研究[21]报道,SLE病人单核细胞TLR4表达水平显著低于健康对照组,TLR2表达水平与健康对照组相比差异无统计学意义。另有研究[22]显示,SLE病人活动期、稳定期血清TLR4表达水平显著高于健康对照组。而本研究旨在通过流式细胞术探究TLR2、TLR4在SLE病人外周血mDC膜表面的表达情况,及其与SLE病人各项临床指标之间的关联以及血清IL-10、IL-17A表达水平,进一步探讨TLR2、TLR4在SLE发病机制中的作用。
本研究发现SLE活动组mDC-TLR2表达率显著高于对照组和稳定组,提示TLR2可能在SLE疾病发病与病情进展中起到重要作用,这一结果与刘昱[23]在SLE病人CD4+T细胞TLR2表达水平研究中的结果一致。本研究中SLE活动组mDC-TLR4表达率显著高于对照组, 差异有统计学意义;稳定组mDC-TLR4表达率高于对照组,但差异无统计学意义,与张文兰等[24-25]的研究结果基本一致,不同之处是本研究中活动组mDC-TLR4表达率高于稳定组,但差异无统计学意义,分析其原因可能是检测方法不同及所测细胞种类不同。本次研究结果提示mDC-TLR2、mDC-TLR4可能在SLE发病过程中起到重要作用,并可能作为SLE药物治疗的潜在靶点,为研制TLRs阻断剂治疗SLE提供一定理论支持。IL-10具有刺激免疫反应和抑制免疫反应的双重作用,在调节免疫反应过程中起到重要作用[26]。IL-17A是IL-17家族具有代表性的成员,其可以诱导多种促炎细胞因子产生,后者可募集B细胞等多种效应细胞,诱导产生大量自身抗体,激活免疫反应。在本研究中,我们发现SLE活动组及稳定组血清IL-10、IL-17A水平显著高于对照组,与以往多项研究[27-29]结果一致,提示IL-10、IL-17A可能参与SLE发病。笔者将SLE病人mDC-TLR2、mDC-TLR4表达率与实验室指标进行相关性分析发现,SLE病人血小板计数与mDC-TLR4表达率呈正相关; 血清IgG水平、24 h尿蛋白定量与mDC-TLR2表达率呈负相关; 抗Sm抗体与mDC-TLR2表达率呈正相关,表明TLR2在SLE发病中起到一定作用。
综上所述,SLE是一种多因素参与的全身性自身免疫病,可引起多器官损害,研究mDC-TLR2、mDC-TLR4在SLE病人中的表达情况有利于对SLE发病机制的进一步探索,有望提供研制TLRs阻断剂治疗SLE的理论可能性,对完善SLE病人的靶向治疗、改善预后,具有重要的临床意义。







 DownLoad:
DownLoad: