-
乳腺癌是女性常见恶性肿瘤和死亡原因之一,中国每年新发病例超过268.6万,占女性新发癌症的15.0%,发病率和死亡率均位居所有恶性肿瘤的第6位,严重威胁女性身心健康[1]。其中,三阴性乳腺癌(triple negative breast cancer,TNBC)是一种特殊类型的乳腺癌,约占所有乳腺癌的15%[2],是指雌激素受体(estrogen receptor,ER)、孕激素受体(progestogen receptor,PR)和人表皮生长因子受体2(human epidermal growth factor receptor-2,Her-2)均为阴性的乳腺癌[3]。TNBC发病年龄轻、侵袭性强、复发早、远处转移率高,其侵袭转移的分子机制研究成为关注重点。
缝隙连接蛋白(connexin,Cx)通过介导的细胞间通信广泛参与人体多种生物学事件的发生,诸如生物电活动的同步、组织分化发育、细胞生长及凋亡、代谢及营养物质传输等[4]。Cx的异常可导致包括肿瘤在内的多种疾病的发生[5-10]。人类乳腺组织中主要表达Cx26和Cx43[11],Cx26的下调或缺失一度被认为是乳腺癌发生进展过程中的重要分子事件[12-13],并且Cx26在淋巴结转移灶中的高表达被认为与预后不良密切相关[14-15]。Cx功能的发挥与多条信号通路有关[16-17],其中核转录因子κB(nuclear factor κB, NF-κB)是一种可与免疫球蛋白κ轻链基因增强子κB序列特异性结合的核蛋白因子,它在自身免疫性疾病、肿瘤(包括乳腺癌)等人类疾病的发生发展中发挥重要作用[18]。本文通过免疫组织化学方法探讨Cx26和NF-κB在TNBC原发灶和区域淋巴结转移灶中的表达,分析二者相关性及其与临床病理特征之间的关系。现作报道。
HTML
-
选取2010-2014年我院手术切除并经术后病理和分子分型确诊为TNBC的病人石蜡标本共计49例,其中无区域淋巴结转移者22例(A1组),有区域淋巴结转移者27例(A2组);将27例淋巴结转移灶作为B组。TNBC诊断标准参考第12届St.Gallen共识中的免疫组化分子分型标准[19];对Her-2基因是否扩增的判断,依据乳腺癌HER2检测指南(2014版)[20],即肿瘤细胞ER(-)、PR(-)、Her-2(-或+)为TNBC。病人均为女性,年龄23~69岁,中位年龄47岁;组织学分级Ⅰ~Ⅱ级28例,Ⅲ级21例;pTNM分期Ⅰ~Ⅱ期30例,Ⅲ~Ⅳ期19例。病人术前均未接受任何治疗,且具有完整临床资料。
-
小鼠抗人单克隆抗体Cx26购于Invitrogen公司,小鼠抗人NF-κB p65单克隆抗体购于Santa Cruz公司,SP试剂盒和DAB显色试剂盒购自中国福州迈新生物技术开发公司。
-
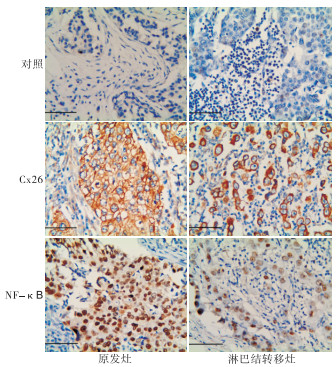
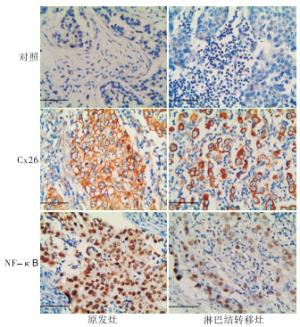
76例石蜡切片脱蜡水化,用柠檬酸缓冲液(pH 6.0)电炉上加热进行抗原修复,然后进行免疫组织化学SP法染色,具体步骤严格按照试剂盒说明书进行。用PBS代替一抗作为阴性对照。光学显微镜下观察阳性染色及细胞定位,并拍照。每张切片在高倍镜视野下观察至少5个视野。Cx26阳性定位于细胞膜或细胞质,细胞内出现黄色颗粒为阳性细胞,阳性细胞≥5%为阳性表达,<5%为阴性表达[21];NF-κB阳性定位于细胞核或细胞质,细胞内出现清晰的淡黄色至棕褐色颗粒者为阳性表达,阴性为细胞核和细胞质均不染色。
-
采用χ2检验、Fisher′s确切概率法和Spearman相关分析。
1.1. 一般资料
1.2. 方法
1.2.1. 主要试剂
1.2.2. 免疫组织化学SP法染色及结果判定
1.3. 统计学方法
-
Cx26蛋白主要分布于乳腺癌细胞的细胞膜,少量见于细胞质,多呈棕黄色颗粒分布;NF-κB主要定位于细胞核,多呈棕褐色染色(见图 1)。原发灶A组的Cx26整体阳性表达率为65.31%,其中A1组阳性表达率为40.91%,低于A2组的85.19%(P < 0.01);原发灶A组的NF-κB整体阳性表达率为53.06%,其中A1组阳性表达率为36.36%,低于A2组的66.67%(P < 0.05)(见表 1)。
分组 n Cx26 χ2 P NF-κB χ2 P 阳性 阴性 阳性 阴性 A1组 22 9 13 8 14 A2组 27 23 4 10.49 <0.01 18 9 4.47 <0.05 合计 49 32 17 26 23 -
淋巴结转移灶B组中Cx26和NF-κB的阳性表达率分别为81.48%(22/27)和77.78%(21/27)),与原发灶A组的65.31%和53.06%比较差异均无统计学意义(P>0.05)。但在亚组分析中发现,B组Cx26和NF-κB的阳性表达率均高于A1组中表达(40.91%和36.36%)(P < 0.01)(见表 2)。
分组 n Cx26 χ2 P NF-κB χ2 P 阳性 阴性 阳性 阴性 A1组 22 9 13 8 14 B组 27 22 5 8.59 <0.01 21 6 8.61 <0.01 合计 49 31 18 29 20 -
Cx26蛋白的表达与TNBC病人的绝经状态、淋巴结是否转移有关(P < 0.01),表现为绝经前和有淋巴结转移者表达更高,而与病人的年龄、肿瘤大小、组织学分级和pTNM分期无关(P>0.05)。NF-κB蛋白的表达与pTNM分期和淋巴结是否转移有关(P < 0.01和P < 0.05),表现为分期越高和有淋巴结转移者表达更高,而与病人年龄、绝经状态、肿瘤大小和组织学分级无关(P>0.05)(见表 3)。
n Cx26 χ2 P NF-κB χ2 P - + - + 年龄/岁 <35 2 0 2 — >0.05* 1 1 — >0.05* ≥35 47 7 30 22 25 绝经状态 绝经前 27 5 22 6.95 <0.01 12 15 0.15 >0.05 绝经后 22 12 10 11 11 肿瘤大小/cm ≤5 45 16 29 0.02△ >0.05 21 24 0.16△ >0.05 >5 4 1 3 2 2 组织学分级/级 Ⅰ~Ⅱ 28 11 17 0.61 >0.05 12 16 0.44 >0.05 Ⅲ 21 6 15 11 10 pTNM分期/期 Ⅰ~Ⅱ 30 12 18 0.96 >0.05 19 11 8.35 <0.01 Ⅲ 19 5 14 4 15 淋巴结转移 阴性 22 13 9 10.49 <0.01 14 8 4.47 <0.05 阳性 27 4 23 9 18 *示Fisher′s确切概率法;△示校正χ2值 -
Cx26和NF-κB在TNBC原发灶中的表达无明显相关性(P>0.05),在淋巴结转移灶组织中两者表达呈明显正相关关系(r=0.663,P < 0.01)。
2.1. Cx26和NF-κB在TNBC原发灶中的表达
2.2. Cx26和NF-κB在TNBC淋巴结转移灶中的表达及其与原发灶的比较
2.3. Cx26和NF-κB表达与TNBC临床病理特征的关系
2.4. Cx26和NF-κB在TNBC原发灶和淋巴结转移灶中表达的相关性
-
Cx是多基因家族编码的一类结构相似而分子量不同的蛋白质,可以通过缝隙连接(gap junction, GJ)依赖性或非GJ依赖性途径发挥肿瘤调控作用[10]。人类乳腺组织中主要表达Cx26和Cx43[11]。早期研究发现Cx26在乳腺癌中具有抑癌作用,因为在多种乳腺肿瘤细胞系上它是低表达或缺失的,并且Cx26蛋白的表达减弱或缺失与乳腺癌发生进展密切相关[12-13]。然而后续研究发现,Cx26在包括乳腺癌在内的某些肿瘤晚期阶段出现高表达,并且与不良预后相关[14-15]。同时有资料显示肿块直径较大的肺癌原发灶Cx26表达缺失,而转移淋巴结中Cx26表达明显上调[22]。基于以上研究结果间的差异,有研究者推测,Cx可能是一种条件性抑癌因子,肿瘤发生发展是多阶段的复杂生物学行为,而Cx蛋白可能不能在所有阶段都使肿瘤得到抑制[10]。与此同时,我们认为肿瘤的异质性导致的这种差异也不能除外,因此本文以TNBC这一亚型乳腺癌为研究对象,通过组织学资料研究Cx26在原发灶和区域淋巴结转移灶中的表达,评估Cx26在TNBC中的表达及临床意义。
本研究结果表明, 在不同组别之间的比较中,Cx26阳性表达率在原发灶A1组与A2组、原发灶A1组与淋巴结转移灶B组间差异均有统计学意义,即Cx26在有淋巴结转移的TNBC原发灶及其转移灶中高表达。临床病理特征相关性分析进一步提示Cx26表达与淋巴结转移、绝经状态相关,表现在淋巴结转移的病人中Cx26表达阳性表达率高,说明其参与了TNBC的浸润转移,而绝经前的TNBC病人预后往往较绝经后差[23],结合本研究结果,这类病人多高表达Cx26,高表达的Cx26促进了肿瘤细胞的侵袭和转移,是预后不良的重要指标。既往的资料也支持这一观点,如有研究[15]报道乳腺癌细胞表达的Cx26可以通过与淋巴管内皮细胞表达的Cx43形成异源性GJ通道进而促进淋巴管侵袭转移。同时研究[24]也发现在恶性黑色素瘤的癌巢中和淋巴管内皮细胞中都有Cx26表达,两种不同组织形成的GJ增加了癌细胞的转移。由此可见,本研究在TNBC这一独特亚型乳腺癌组织学中明确了Cx26在淋巴结转移中的作用,为Cx在不同种类的恶性肿瘤以及肿瘤的不同阶段扮演不同的角色这一观点提供了更多的实验证据。
研究[25]发现,Cx家族成员调控乳腺癌细胞的生长与一些转录因子及其信号通路有关。同时近期发表在权威杂志《Nature》上的一篇报道[7]也证实脑转移瘤细胞会通过Cx43介导的GJ通道传递第二信使cGMP,从而激活下游NF-κB通路促进肿瘤细胞的生长和化疗耐药,因此我们对NF-κB的表达及其与Cx26的相关性一并进行了研究。研究结果发现NF-κB表达有统计学差异的组别与Cx26完全一致,与pTNM分期及淋巴结转移相关,并且在TNBC淋巴结转移灶的相关性分析中与Cx26表达呈正相关关系,这些提示Cx26促进TNBC浸润转移的机制可能与NF-κB信号通路有关。本研究为Cx26与NF-κB两者在TNBC组织中表达的相关性提供了组织学证据,并明确了两者在介导淋巴结转移中的作用。当然Cx26如何通过激活NF-κB信号通路发挥促进肿瘤转移的作用,是GJ依赖性或非GJ依赖性等均有待于进一步的研究。
综上所述,Cx26和NF-κB在有淋巴结转移的TNBC原发灶及其淋巴结转移灶中高表达,且在转移灶中二者表达呈正相关关系,提示两者共同参与TNBC的浸润转移,两者联合检测有望预测TNBC转移复发及评估临床预后。未来对其作用机制及信号通路的进一步揭示,可能为TNBC的治疗提供新靶点和新策略。






 DownLoad:
DownLoad: