-
感音神经性耳聋(sensorineural hearing loss, SNHL)的病因有很多, 主要是各种内耳的先天及后天性病变[1], 目前人工耳蜗植入术(cochlear implantion, CI)是治疗重度、极重度SNHL的主要方法[2], 因此需要通过影像学检查诊断并筛选出CI手术的适应证, 并在术前排除该手术的各种禁忌证。目前在耳聋原因的检查中, 除了临床专科检查, 最常见的检查是颞骨高分辨率CT(HRCT)及MRI水成像检查, CT对耳部骨性结构、各种钙化骨化等显示好, MRI对软组织以及颅内结构的病变显示好, 特别是MRI水成像技术的应用, 使内耳膜迷路精细结构的显示成为可能。使用单一的检查方法可能会造成疾病的漏诊、误诊, 从而影响治疗。因此, 本文旨在探讨使用颞骨HRCT扫描联合MRI水成像技术在SNHL病因诊断的临床应用价值, 提高诊断的准确性。
HTML
-
收集本院2018年1月至2019年6月临床表现为SNHL的病人75例, 其中男41例, 女34例, 年龄1~56岁。均进行CT及MRI检查。
-
CT检查:采用GE 256排Revolution CT能谱扫描仪。扫描参数:高分辨螺旋扫描(HiRes Helical), 管电压100 kV, 管电流150 mA, 层厚及层间距0.625 mm, 螺距1.375:1, HD Boneplus算法, 扫描结束分别对两侧乳突进行靶重建, FOV 7.0。MRI检查:采用Philips Achieva 3.0T双梯度超导型磁共振扫描仪, 头部8通道相控阵专用线圈。扫描序列及参数:首先行常规颅脑扫描, T1WI-tse-tra (TR 500 ms, TE 25 ms, FOV 23 cm×23 cm, Matrix 512×512, 层厚6 mm, NEX 2), T2WI-tse-tra (TR 4 000 ms, TE 93 ms, FOV 23 cm×23 cm, Matrix 512×512, 层厚6 mm, NEX 2), 主要是为了观察有无颅内病变。然后行T1WI-se-tra(TR 450 ms, TE 17 ms, FOV 17 cm×17 cm, Matrix 512×512, 层厚2 mm, NEX 2), T2WI-se-cor-fs (TR 3 500 ms, TE 74 ms, FOV 17 cm×17 cm, Matrix 512×512, 层厚2 mm, NEX 3)内耳超薄层无间距扫描。3D-CISS序列的参数[TR 5.98 ms, TE 2.67 ms, FOV 18 cm×18 cm, Matrix 512×512, 有效层厚0.5 mm, 体素大小0.5 mm×0.5 mm×0.4 mm, NEX 1, 翻转角(FA)采用70°, 扫描时间308 s]。在相应的工作站上进行图像后处理, 采用MPR、VR、MIP等重建技术对内耳结构进行多方位、多角度观察。
-
采用t检验和Kappa检验。
1.1. 一般资料
1.2. 检查方法及参数
1.3. 统计学方法
-
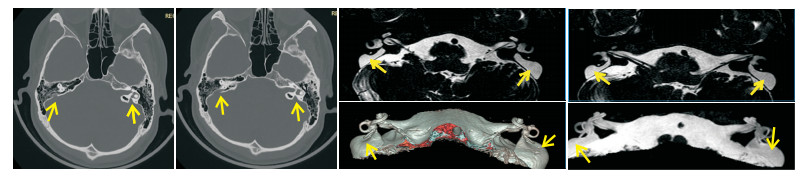
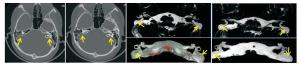
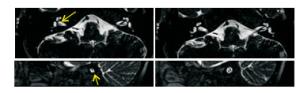
所有检查者颅内均未见病变, 排除颅内病变导致的听力障碍。75例中, 45例耳结构异常, 30例未见异常。MRI上, 30例(60耳)正常内耳不同侧别、不同性别间主要解剖结构测量结果差异均无统计学意义(P>0.05)。45例耳结构异常:(1)半规管畸形3例(3/45, 6.67%), CT检出2例, 表现为骨迷路的畸形; MRI检出3例, 表现为膜迷路的畸形, 1例左耳外半规管缩短, 1例右耳后半规管局部连续性中断, 1例右耳外半规管部分缺损。CT及MRI两种方法检出半规管畸形具有很高的一致性(Kappa=0.825)。(2)耳蜗畸形4例:包括Michel畸形1例(1/45, 2.22%), CT与MRI均能检出, 表现为所有耳蜗及前庭结构完全缺如; Mondini畸形3例(3/45, 6.67%), CT与MRI均能检出, 表现为耳蜗只显示一圈半(1.5圈), 且耳蜗直径缩小, 耳蜗顶周与中周相互融合成囊状, 底周未见明显异常, 其中1例Mondini畸形伴有两侧前庭的扩大。CT及MRI两种方法检出耳蜗畸形有很高的一致性(Kappa=1.000)。(3)前庭导水管畸形9例(9/45, 20.00%), CT检出8例, MRI均能检出, CT表现为前庭导水管扩大(导水管内径的宽度>1.5 mm), 呈"喇叭口"状。MRI表现为囊状扩张的内淋巴囊及扩张的内淋巴管。CT及MRI两种方法检出大前庭导水管综合征有很高的一致性(Kappa=0.892)(见图 1)。(4)内听道畸形4例(4/45, 8.89%), MRI检出1例, CT均能检出, 3例表现为两侧内听道狭窄(< 3 mm), 1例两侧内听道呈喇叭口样扩大(>7 mm)。CT及MRI两种方法检出内听道畸形的一致性较差(Kappa=0.380)。2例外耳道异常(2/45, 4.44%), CT均能检出, 表现为外耳道内异常高密度影, MRI均未检出。(5)蜗神经发育不良18例(18/45, 40.00%), CT均未检出, MRI均能检出, 表现为蜗神经支缺如、未见显示5例(见图 2); 蜗神经支纤细13例, 明显细于同层的面神经支。(6)内听道内血管发育异常5例(5/45, 11.11%), CT均未检出, MRI均能检出, 其中神经血管粘连1例, 神经血管压迫4例。
-
本组75例SNHL中, 45例CT和/或MRI检查耳部存在异常, 占60.00%(45/75), 由此可见内耳病变是SNHL的最重要原因。目前CI术是治疗重度、极重度SNHL的主要方法, 需要通过影像学检查诊断并筛选出CI手术的适应证和禁忌证是非常重要的。
颞骨内的解剖结构精细复杂, 体积小, 形态多样, CT是评价颞骨的主要影像学方法, 并且一直是作为评估患耳CI指征的初步筛选方法[3]。CT对骨质结构有良好的显示能力, 是扫描精细骨结构和鉴别内耳病理性钙化和硬化的首选方法。颞骨HRCT薄层扫描有高分辨率、低辐射剂量、扫描时间短等优点, 可清晰显示内耳骨迷路、骨性面神经管、前庭导水管以及外耳道等情况, 并可显示骨质受侵破坏情况, 帮助诊断内耳病变, 而多平面重建、最大密度投影及容积再现技术等多种后处理不仅可以直观、立体地显示听小骨、内听道等细小结构, 还可模拟CI手术路径, 对CI术前评估、术中操作均有重要价值[4]。本次研究颞骨HRCT检出半规管畸形2例, 耳蜗畸形4例, 大前庭导水管综合征8例, 内听道狭窄4例, 外耳道病变2例, 诊断准确率高。而HRCT对于内听道的神经、血管的显示欠佳, 常不能做出正确的判断, 从而影响诊断及治疗。
主要结构/mm 侧别 性别 左(n=30) 右(n=30) t P 男(n=16) 女(n=14) t P 内听道直径 5.60±0.10 5.55±0.11 0.94 >0.05 5.58±0.14 5.48±0.12 0.95 >0.05 前半规管直径 1.25±0.15 1.32±0.14 0.91 >0.05 1.29±0.18 1.22±0.15 0.87 >0.05 后半规管直径 1.35±0.24 1.40±0.25 0.90 >0.05 1.28±0.22 1.30±0.26 0.92 >0.05 外半规管直径 1.18±0.03 1.17±1.05 0.95 >0.05 1.16±0.05 1.15±1.04 0.96 >0.05 椭圆囊与球囊的垂直径 5.25±0.67 5.31±0.60 0.89 >0.05 5.35±0.57 5.26±0.68 1.20 >0.05 椭圆囊与球囊的横径 3.26±0.84 3.34±0.78 0.97 >0.05 3.35±0.76 3.32±0.75 0.89 >0.05 耳蜗高度 4.75±0.10 4.70±0.09 0.96 >0.05 4.71±0.18 4.69±0.22 0.94 >0.05 蜗底直径 7.25±0.08 7.30±0.05 0.99 >0.05 7.32±0.06 7.28±0.08 0.91 >0.05 蜗管直径 2.02±0.18 1.85±0.22 0.47 >0.05 2.00±0.16 1.98±0.15 0.49 >0.05 耳蜗弧长 22.50±1.85 21.9±3.06 0.83 >0.05 22.3±2.88 21.5±3.03 0.93 >0.05 耳蜗容积Δ 94.00±6.80 94.80±5.90 0.57 >0.05 96.50±7.80 93.20±8.90 0.63 >0.05 面神经直径 1.05±0.17 1.09±0.22 0.98 >0.05 1.05±0.19 1.03±0.21 0.89 >0.05 前庭上神经直径 1.06±0.21 1.03±0.22 0.99 >0.05 1.05±0.18 1.04±0.20 0.88 >0.05 蜗神经直径 1.08±0.18 1.04±0.15 0.97 >0.05 1.06±0.16 1.05±0.19 0.92 >0.05 前庭下神经直径 0.62±0.22 0.59±0.23 0.68 >0.05 0.60±0.18 0.58±0.21 0.57 >0.05 Δ示容积单位为mm3 随着3.0T高场强MRI的应用及后处理技术近年来的完善, 特别是内耳MRI水成像技术的应用, 使内耳膜迷路的显示成为可能。3D-CISS序列使内耳及桥小脑角区流动慢的液体如淋巴液、脑脊液、慢血流等呈表现为明显的高信号, 从而与周围骨性结构的低信号区分开来, 是内耳影像学检查的理想水成像序列[5]。3D-CISS序列由于不需要钆对比剂, 因此不存在肾源性全身毒性的风险。具有高信噪比、高对比度(脑脊液与周围组织)、高空间分辨率、薄层扫描(最小有效层厚可达0.5 mm)、成像时间短等优点。3D-CISS序列结合MPR、MIP、VR重建法能够清晰地显示双侧面神经、蜗神经、前庭上神经、前庭下神经的走行、形态及信号。清晰地显示3个膜半规管、前庭、耳蜗及内听道的结构。四条神经均为低信号, 半规管、前庭、耳蜗管及内听道内的脑脊液显示为高信号。
本次研究测量了内耳主要结构的正常值, 并对不同侧别及性别的内耳进行比较, 对于巩固对内耳的认识或进一步研究阐明病变情况具有重要意义。MRI显示内耳的骨性结构较差, 而MRI软组织分辨率高, 耳蜗的早期纤维化、内耳畸形、内耳肿瘤、蜗神经前庭神经发育情况以及颅内病变在MRI上能很好的表现出来。耳蜗早期纤维化在CT上很难发现, 文献[6]报道CT漏诊率可达57%, 而MRI可以发现早期的纤维化与软组织的异常。前庭导水管扩大引起的SNHL较为常见, 本组8例MRI能均显示, CT显示7例, 1例假阴性。MRI除了能显示CT上的前庭导水管喇叭口样扩大, 还能直接显示膜迷路的液体, 更能反映其形态的变化, 是更准确的影像诊断手段。LOPEZ-ESCAMEZ等[7]认为内淋巴管及内淋巴囊的积水程度与感音神经性听力损失程度有关。本次研究CT发现内听道狭窄4例, 而内听道内的神经发育正常, MRI上发现蜗神经发育不良18例, 而内听道均正常, 因此通过CT上内听道是否狭窄来判断神经是否发育不良是不准确的。MRI能够很好地显示内听道内的神经、异常血管襻。蜗神经的发育异常多见, 单纯的前庭神经发育异常比较少见。3D-CISS序列能够很好显示SNHL病人的前庭蜗神经发育情况, 可用于评估前庭蜗神经是否存在轴索丢失的影像学征象。有研究[8-9]显示, 蜗神经发育异常的病人有50%可伴有前庭神经的发育异常, 并且约11%蜗神经发育异常的病人是有耳聋的家族史的[10]。本次研究75例中有36例内听道内见异常血管襻穿行, 有5例异常血管襻压迫粘连到周围面、听神经, 引起听力下降及面肌痉挛。正常人内听道内也可有异常血管襻穿行。MRI技术可用于人工耳蜗手术病人的术前评估[11]。PRABHU等[12]研究了3D-CISS成像对人工耳蜗植入病人的术前预测价值, 比较了术前MRI表现及手术中的情况。发现术前MRI表现与术中发现的有关整个耳蜗通畅和异常情况, 以及对耳蜗、前庭和面神经病理学的诊断和内耳道异常之间有高度的相关性, 术前可以通过重建检测出耳蜗纤维化以及内耳迷路各种畸形, 从而在一定程度上排除一些绝对或相对手术禁忌证, 提高手术的成功率[13-14]。
总之, HRCT成像可清晰显示内耳骨性结构, 3D-CISS MRI水成像可清晰地显示内耳膜迷路、神经、血管的形态结构。两种检查技术各有优势, 在SNHL的病因的检查中有重要意义, 帮助诊断各种内耳疾病, 充分降低漏诊误诊率, 为CI等手术提供重要信息, 大大提高手术的准确性。






 DownLoad:
DownLoad:
