-
结直肠癌(colorectal cancer,CRC)是全世界常见的消化道恶性肿瘤之一。相关研究数据表明,近几年我国CRC的发病率和死亡率均保持上升趋势[1]。早期肿瘤病人可通过手术切除获得良好预后,其5年生存率可超过90%。然而多数CRC确诊时已为中晚期,无法达到理想的治疗效果,5年生存率不到10%[2]。CRC中常常有代谢失常的表现[3]。泛醌-细胞色素c还原酶复合物核心蛋白1(ubiquinol cytochrome c reductase core protein 1,UQCRC1)是线粒体复合体Ⅲ的重要结构亚基之一,参与组成线粒体氧化呼吸链,对维持正常线粒体功能和细胞代谢有着基础性作用[4]。国内外大量研究表明,UQCRC1在人类肿瘤中存在表达失调[5]。癌胚抗原(carcinoembryonic antigen,CEA)是目前应用最广泛的肿瘤标志物之一,经常用于肠癌的检测中。但因其特异性与敏感性欠佳,常与其他肿瘤标志物联合使用[6]。本研究通过检测CRC根治术后的组织标本中UQCRC1的表达,分析其和血清CEA的相关性,初步探讨UQCRC1在结肠癌发生、发展过程中的作用及意义。
HTML
-
随机选取2017-2018年蚌埠医学院第一附属医院初诊CRC手术切除得到的组织标本52例,并收集所选病例术前血清CEA值。其中男28例,女24例,年龄20~80岁。肿瘤大小:≤5 cm者27例,>5 cm者25例;组织学分型:腺癌49例,黏液癌3例;组织学分级:中分化及高分化共36例,低分化16例;TMN分期:Ⅰ期2例,Ⅱ期22例,Ⅲ期20例,Ⅳ期8例;并从其中选取37例临近癌旁组织作对照分析。纳入标准:所有病人均已完善相关检查(肠镜活检、临床病理切片等)确诊为原发性CRC,且为初诊;术前未接受化疗、放疗、靶向及其他特殊抗肿瘤治疗;所有病人均已在术前完善血清学CEA检测。排除标准:合并其他系统肿瘤;合并急慢性严重炎症、自身免疫性病;临床资料不全者。诊断及分期标准:参照《中国CRC诊疗规范(2017年版)》[7]中提到的诊断标准及TNM分期、组织学分级等。
-
本研究已通过本院伦理委员会批准。抗体UQCRC1购自美国Abcam有限公司(产品编号ab125882),免疫组织化学采用SP法染色。按照试剂盒说明书进行染色程序,主要步骤包括:石蜡切片二甲苯脱蜡,梯度乙醇脱水;自来水、蒸馏水各冲洗3遍;高压抗原修复2 min;过氧化物酶阻断10~15 min;蒸馏水冲洗3遍,PBS冲洗;加一抗温箱孵育1 h,PBS冲洗3遍;滴加二抗,温箱孵育30 min,PBS冲洗3遍;滴加DAB显色3~5 min,自来水冲洗;苏木精复染,水洗返蓝;常规脱水透明,中性树胶固定封片。
-
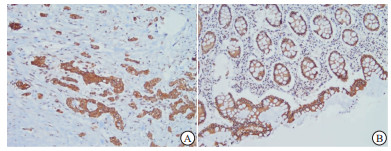
以细胞质出现棕黄色颗粒为阳性。显微镜下观察免疫组织化学染色结果,每张切片由本院2名病理科医生采用下述方法评定[8]:标志物根据显色深浅得到染色强度:0分(阴性)、1分(弱)、2分(中)、3分(强);根据阳性染色区域相对于整个癌区百分比得到染色程度: < 5%为0分、5%~25%为1分、>25%~50%为2分、>50%~75%为3分、>75%为4分。其结果为两项得分乘积:最终得分≤2分为阴性,最终得分≥3分为阳性。血清CEA值参考值范围为0~5 ng/mL, ≥5 ng/mL即为阳性。
-
采用χ2检验、Spearman等级相关性分析。
1.1. 一般资料
1.2. 实验方法
1.3. 结果判定
1.4. 统计学方法
-
UQCRC1主要表达于细胞质(见图 1)。本次研究结果显示,52例癌组织中有16例(30.8%)UQCRC1低表达;37例癌旁正常组织中UQCRC1呈低表达的有4例(10.8%),在α < 0.05的水准上,两者差异具有统计学意义(χ2=4.94,P < 0.05)。Spearman等级相关性分析结果提示UQCRC1的表达与组织性质显著相关(r=-0.236, P < 0.05)。CRC病人术前血清CEA阳性率为40.4%(21/52)。
-
CRC组织中UQCRC1的表达与淋巴结转移情况有关(P < 0.05),在病人不同性别、年龄、肿瘤大小、分化程度、临床分期上的表达差异均无统计学意义(P>0.05) (见表 1)。
临床病理参数 n UQCRC1 χ2 P 低表达 高表达 性别 男
女28
247(25.0)
9(37.5)21(75.0)
15(62.5)0.95 >0.05 年龄/岁 ≤60
>6027
2510(37.0)
6(24.0)17(63.0)
19(76.0)1.04 >0.05 肿瘤大小/cm ≤5
>527
259(33.3)
7(28.0)18(66.7)
18(72.0)0.17 >0.05 分化程度 低分化
中+高分化16
363(18.8)
13(36.1)13(81.3)
23(63.9)0.86* >0.05 临床分期 Ⅰ+Ⅱ
Ⅲ+Ⅳ24
285(20.8)
11(39.3)19(79.2)
17(60.7)2.07 >0.05 淋巴结转移 是
否22
3010(45.5)
6(20.0)12(54.5)
24(80.0)3.86 < 0.05 *示校正χ2值 -
52例结肠癌组织中,UQCRC1表达阴性同时CEA表达阳性者占62.5%(10/16)。运用Spearman等级相关分析得知,在α < 0.05的检验水准上,CRC病人癌组织中UQCRC1表达水平与术前血清CEA值呈显著负相关(r=-0.300)(P < 0.05)(见表 2)。
UQCRC1表达 n CEA表达 r P 阳性 阴性 阴性
阳性16
3610
116
25-0.300 < 0.05
2.1. CRC组织中UQCRC1的表达和血清CEA阳性率
2.2. CRC组织中UQCRC1的表达与临床及病理参数的关系
2.3. CRC组织UQCRC1和血清CEA表达水平的相关性
-
CRC是全世界绝大多数国家的一个健康问题。据报道,全世界每年有超过100万人罹患CRC,遗传和环境因素是CRC发生的重要条件[9]。在我国,CRC发病率逐年上升,且随着一些危险因素如人口老龄化、不良饮食习惯等蔓延,其死亡率也处于明显的上升趋势[10],居民生命健康受到严重威胁。尽早诊断和判断癌变组织恶性程度对提高CRC病人的生存质量至关重要。
UQCRC1是一种核DNA编码蛋白,是参与合成线粒体呼吸链复合体Ⅲ的11个重要亚基之一。UQCRC1在胚胎存活和发育中起着关键作用,SHAN等[11]的实验发现敲除杂合子小鼠中的UQCRC1导致胚胎致死。而关于UQCRC1的表达与恶性肿瘤中的关系,KULAWIEC等[12]发现74%的乳腺癌病人UQCRC1阳性,仅34%的卵巢腺癌病人UQCRC1表达。后陆续有关于其在食管癌、胰腺导管腺癌中上调,在贲门癌、胃癌中表达下调的报道[13-14]。从目前考虑的机制上看,不可忽视的是UQCRC1表达与线粒体的关系。研究[11]证实UQCRC1对维持酵母中复合体Ⅲ的完整性至关重要。有关实验[15-16]显示,上皮来源的细胞和小鼠精母细胞中UQCRC1的缺失可导致线粒体功能障碍。YI等[17]的研究指出,UQCRC1有助于在心肌损伤期间维持H9c2细胞的线粒体功能。根据这些证据可推测,UQCRC1的异常表达可影响到人类线粒体功能正常状态的维持。正常线粒体功能中断会导致多种人类疾病的发生,其中就包括肿瘤的发生[18]。近年来,线粒体出现在癌症研究中的次数大大增多。线粒体可调节生物代谢,是癌细胞代谢变化的关键调节物[19]。目前线粒体功能障碍在多种癌症的转移进展中皆有报道发现[20],如结肠癌、前列腺癌、胃癌等。线粒体障碍常产生大量活性氧(reactive oxygen species,ROS),促进DNA损伤和遗传不稳定性[21]。ROS是线粒体呼吸链产生的主要来源于复合体Ⅲ的具有损伤作用的副产物[22],ROS失衡是线粒体功能障碍主要机制之一,其在癌症的发生、发展和生存表型中皆发挥重要作用[19, 23-24]。SHAN等[11]实验还发现,UQCRC1缺乏致使小鼠线粒体膜电位和ATP产量下降,而ROS产量增加,可能原因在于UQCRC1的缺乏导致复合物Ⅲ活性的降低。
本实验研究结果显示,UQCRC1在癌组织中的表达下调率(30.8%)高于癌旁正常组织(10.8%),差异有统计学意义(P < 0.05)。UQCRC1参与组成线粒体氧化呼吸链,推测在结直肠细胞中UQCRC1的下调表达使细胞中的线粒体出现功能障碍,细胞能量代谢发生改变,进而导致肿瘤的发生发展。进一步分析该蛋白表达水平与肿瘤的大小、分化程度、临床分期以及淋巴结转移的关系,发现CRC组织中UQCRC1的下调表达与肿瘤淋巴结转移有关,进一步提示UQCRC1的存在与CRC侵袭有着密切联系。LI等[25]通过研究证实UQCRC1表达下调与血管内皮生长因子-C(vascular endothelial growth factor-C,VEGF-C)表达升高之间存在正向相关关系,提出UQCRC1的下调可诱导VEGF-C的表达进而促进淋巴转移。这一考虑基于有关VEGF-C通过诱导淋巴管的生成促使淋巴结转移事件发生的相关报道[26]。
CEA是一种高分子糖蛋白,在人类的结肠、胃、汗腺等组织器官中存在。CEA在细胞的聚集、黏附、凋亡等过程中发挥作用,其与肿瘤的恶性程度密切相关。CEA增高可发生在多种恶性肿瘤中如胃肠道癌、乳腺癌、胰腺癌等,其中以结肠癌显著[27-28]。研究[29]显示CEA在CRC病人中的阳性率在40%~50%之间。此次研究结果显示,CRC病人术前CEA阳性率为40.4%,UQCRC1表达与血清CEA表达存在负相关性。这种负向关系考虑原因为:在结肠细胞中UQCRC1的表达下调或缺失,使得细胞线粒体失去正常功能,细胞代谢环境改变使得组织器官更易受到损伤,从而导致肠道细胞癌变。随着CRC的发生,CEA含量增多,充分发挥了其在肿瘤细胞免疫逃避及细胞聚集、黏附中的重要作用,使肿瘤细胞更易向周围组织器官扩散和转移[30],进一步促进了癌症的发展,使得UQCRC1的表达进一步下调。CEA作为标志物在肿瘤的检测中应用广泛,但因独立检测缺乏特异性,增加指标可使阳性可信度增加。本实验结果显示,CRC组织中UQCRC1表达阴性者其血清CEA阳性率明显高于UQCRC1表达阳性者,表明联合两种指标检测有助于评估结直肠肿瘤的状态。
综上所述,CRC中UQCRC1的表达下调与CRC的发生发展有密切关系,联合UQCRC1和CEA两个指标检测可为CRC的诊治及病情评估提供一定理论依据。







 DownLoad:
DownLoad: