-
胰腺脂肪沉积(pancreatic fat deposition,PFD)是指胰腺腺泡或胰岛细胞中有三酰甘油累积,或胰腺实质被脂肪组织替代[1]。PFD的发生可直接损害胰岛β细胞或间接抑制细胞胰岛素信号转导,最终诱发β细胞凋亡引起糖尿病,而糖尿病、肥胖、代谢综合征等又可导致胰腺发生脂肪化,进一步加重病人病情[2-6]。脂肪积聚于胰腺实质胰岛细胞或腺泡细胞是可逆的,称为胰腺脂肪浸润;脂肪细胞积聚于胰腺实质内是不可逆的,称为胰腺脂肪替代[7-8]。由于胰腺脂肪沉积部分为可逆性的,因此定量评价胰腺脂肪含量对于胰腺脂肪沉积的早期诊断、早期干预十分重要,而双能量能谱CT定量分析技术能够克服传统CT的伪影及部分容积效应等弊端、对病灶脂肪含量精准判断,对胰腺脂肪沉积预防、早期诊断具有重要价值。本研探讨双能量能谱CT定量分析技术在PFD中的诊断价值。现作报道。
HTML
-
收集2017年3月至2019年8月65例在我院行腹部CT扫描的病人资料,根据病人病情分为2组。观察组35例,其中男22例,女13例;年龄21~76岁;临床表现为2型糖尿病32例,代谢综合征及肥胖症1例,高胰岛素血症1例,单纯性肥胖1例;纳入标准:糖尿病、高血脂或代谢综合征病人。对照组30例,其中男20例,女10例;年龄26~65岁;纳入标准:排除糖尿病,无血脂异常,无胰腺疾病、无病理性肥胖[体质量指数(BMI) < 24 kg/m2],因体检或其他疾病行腹部CT扫描病人。BMI=体质量(kg)/身高2(m2)。BMI < 24 kg/m2正常,≥24 kg/m2超重,≥28 kg/m2肥胖。
-
病人空腹8 h,并于检查前15~30 min饮用500~1 000 mL清水,使胃和十二指肠充盈。用GEDiscovery CT 750HD扫描机行腹部平扫,病人取仰卧位,扫描范围自膈顶至肋弓下缘,采用GSI模式扫描。扫描参数:螺旋扫描速度每圈0.8 s,螺距:1.375∶1,准直宽度0.625 mm×64;电压为高低能量(140 kVp和80 kVp)0.5 ms瞬时切换,管电流约为550 mA。
-
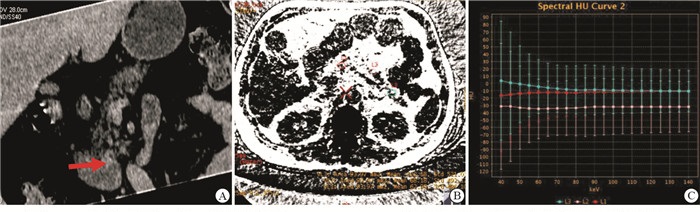
将平扫单能量图像传输至Advanced Workstation(ADW 4.6,GE Health)工作站处理,行脂肪测量及分析。主要测量及分析方法为:在最佳keV值单能量图像上,分别测定胰腺头、体、尾部的CT值,并取均值;在脂肪-水物质图像上,分别测定胰腺头、体、尾部的脂肪含量,并取均值;获得胰腺各部组织的能谱曲线,并做出平均能谱曲线,以直观显示胰腺组织的脂肪含量变化情况。
-
采用t检验、方差分析和q检验。
1.1. 一般资料
1.2. 方法
1.3. 后处理方法
1.4. 统计学方法
-
所有平扫单能量图像传输至GE ADW 4.6工作站处理,进行后处理脂肪测量及分析,随着胰腺实质中脂肪含量的增强,胰腺组织能谱曲线的斜率逐渐降低。典型病例见图 1~3。
-
观察组35例中,BMI为19.03~42.44 kg/m2,8例BMI正常,其余均为超重或肥胖;胰腺平均CT值随着BMI增高呈下降趋势,而平均脂含量值随BMI增高呈上升趋势,差异均有统计学意义(P < 0.01)(见表 1)。观察组胰腺组织的能量CT值及水含量值均显著低于对照组,脂肪含量值显著高于对照组(P < 0.01)(见表 2)。
分组 n CT值/Hu 脂肪含量(脂-水配对)/(mg/mL) BMI/(kg/m2) < 24 8 40.33±4.41 -106.21±43.29 24~ < 28 19 35.35±6.07 -80.45±62.01 ≥28 8 22.71±6.81**## 37.42±53.69**## F — 19.49 15.76 P — < 0.01 < 0.01 MS组内 — 35.124 3 203.463 q检验与BMI < 24 kg/m2比较**P < 0.01,与BMI 24~28 kg/m2比较##P < 0.01 分组 n CT值/Hu 脂肪含量(脂-水配对)/(mg/mL) 水含量(水-脂配对)/(mg/mL) 观察组 35 31.91±6.72 -22.37±64.56 1 182.69±52.17 对照组 30 50.23±4.13 -179.23±8.95 1 305.10±20.65 t — 13.44 14.22 12.76 P — < 0.01 < 0.01 < 0.01
2.1. 图像分析
2.2. 观察组BMI值、胰腺CT值及脂含量值分布情况
-
组织学诊断为公认的PFD诊断金标准,但因活体难以取得目的组织导致临床应用受限。目前临床上用于诊断PFD的手段多为影像学检查,包括腹部超声、CT和MRI[6]。超声检查表现为胰腺回声增强,强于肝脏回声[6, 9],但是超声仅能对PFD进行定性或半定量分析,且胰腺为腹膜后脏器,易受肠道气体干扰。多数学者对MR技术在量化胰腺脂肪含量的应用进行研究,常用的方法[10-12]有MR双回波化学移位成像、频率选择脂肪预饱和技术、磁共振波谱分析等,但效果均不佳。常规CT平扫是检测PFD最普遍的方法,但传统CT采用混合能量X射线,会使CT值产生漂移,且CT值容易受机器型号及性能、伪影及部分容积效应等影响,因此准确性亦有限[13]。以瞬时双keV为核心技术的双能量能谱CT成像是CT成像领域中的一项新技术。能谱CT扫描覆盖范围广,扫描所需时间短,且CT能谱成像将传统的X线混合能量图像通过特定软件和算法分解成不同keV水平的单能量图像,可以获得扫描野内各器官的多种基物质图像(如水、钙、脂肪等)以及能谱曲线,进行物质定量及能谱综合分析[13-15],其优势在于:(1)采用最佳对比噪声比技术,可获得最佳keV的单能量图像,因其具备较高的图像质量和对比噪声比,可消除射线硬化伪影,使CT值更加准确。(2)能谱CT的物质定量分析技术可以对碘、水、脂肪等物质进行两两配对,进行物质定量测定。(3)能谱曲线反映了感兴趣区内组织在40~140 keV不同条件下CT值的连续变化情况,含脂肪组织表现为弓背向上的曲线。本研究采用能谱CT对胰腺组织脂肪的沉积情况进行研究发现,胰腺平均CT值随着BMI增高呈下降趋势,而平均脂含量值随BMI增高呈上升趋势,差异均有统计学意义(P < 0.01);观察组胰腺组织的能量CT值及水含量值均显著低于对照组,脂肪含量值显著高于对照组(P < 0.01)。能谱CT的脂肪-水物质图为PFD的判断提供了一个简单易行的测量方法,为临床早期诊断、早期治疗代谢综合征提供更多有价值的信息。
本研究尚存在以下不足:(1)未能对高血糖、高血脂等不同原因所致的胰腺脂肪化进行分类研究。(2)因样本量较少,未能对PFD程度进行分级。今后将加大样本量,进行更细致的研究。










 DownLoad:
DownLoad: