-
乳腺癌是女性发病率最高的恶性疾病之一,近年来的发病率逐年上升,严重危害了女性的健康。长链非编码RNA(LncRNA)为长度>200个核苷酸的RNA分子,根据互补碱基配对的原理,具有多种二级结构,可与多种蛋白质和核酸特异性结合[1],MicroRNA(miRNA)是一类小的、非编码RNA分子,通过与靶基因mRNA 3′末端非译区(3′-UTR)结合降解该mRNA从而抑制蛋白翻译来调控基因表达[2]。TAVAZOIE等[3]通过干扰miRNA-126,沉默CXCR4,显著降低了乳腺癌细胞生长及增殖,该研究发现LncRNA和miRNA可通过调控CXCR4表达从而调控乳腺癌转移和耐药诱导。本项目组通过生物信息学软件分析发现长链非编码RNA小核仁RNA宿主基因15(LncRNA SNHG15)与miRNA-451a、趋化因子受体4(CXCR4)具有相应的结合序列。猜测SNHG15与CXCR4、miRNA-451a之间具有某种调控作用。本研究旨在探究LncRNA SNHG15、miRNA-451a与CXCR4对乳腺癌细胞的调控作用,以及对其生物学活性的影响。
HTML
-
人乳腺癌细胞株SKBR3购买于中国科学院细胞库,胎牛血清和DMEM高糖培养基购买于Hyclone公司;Trizol试剂、Lipofectamine2000购买于Invitrogen公司;荧光定量聚合酶链反应(qRT-PCR)试剂购买于Takara公司;LncRNA SNHG15干扰片段、miRNA-451a抑制剂/类似物购买于上海吉玛公司,引物由南京金斯瑞生物科技有限公司合成;一抗Vimentin、slug、CXCR4购买于Proteintech以及Affinity公司。
-
用紫杉醇诱导SKBR3产生耐药性,于紫杉醇浓度10 μg/mL、10%胎牛血清的DMEM培养基,37 ℃、5%CO2环境中生长,当细胞生长于对数生长期时,消化细胞进行相关实验处理。
-
SKBR3-PR细胞铺6孔板培养,细胞生长至50%~60%时,配置转染试剂A、B液,将B液加进A液充分混匀后静置20 min,将6孔板旧培养基吸出,用PBS清洗,更换新的10%胎牛血清的DMEM培养基。将配置好的转染试剂加入相应的孔中培养24或48 h。
-
6孔板细胞生长24 h后,用胰酶消化,加入Trizol试剂提取RNA并逆转录,以GAPDH为内参,按照qRT-PCR试剂说明进行PCR反应,按95 ℃ 15 s, 60 ℃ 30 s, 72 ℃ 30 s的反应条件进行循环。反应结果表示为2-△△CT。LncRNA SNHG15、CXCR4、GAPDH引物序列见表 1。
引物名称 上游序列 下游序列 LncRNA SNHG15 GCC TCC CAG TTT CAT GGA CA GCT GAG GTG ACG GTC TCA AA CXCR4 CGT GGA ACG TTT TTC CTG TT AGG TGC TGA AAT CAA CCC AC GAPDH TGT GGT CAT GAG TCC TTC CA CAG CCT CAA GCT CAT CAG CA -
检测SNHG15、miRNA-451a的表达变化对乳腺癌细胞迁移能力的影响,SKBR3-PR细胞铺6孔板培养,当细胞生长至80%~90%时,用10 μL的无菌枪头分别在6孔板的中间以及两侧各1/3处用相同的力道划一道笔直的伤痕,然后吸取旧培养基,更换无血清的DMEM培养基,实验组再加入相应的转染试剂,培养24 h后,在显微镜下观察0 h与24 h的细胞迁移情况。
-
检测SNHG15、miRNA-451a表达对乳腺癌细胞凋亡能力的影响,SKBR3-PR细胞铺6孔板培养,当细胞生长至50%~60%左右时,进行相应的实验组转染处理,培养48 h后,用预冷的PBS冲洗2遍,用不含EDTA的胰酶消化,流式样本处理好后静置15~20 min,上机检测。
-
检测CXCR4以及上皮间质转化(EMT)相关蛋白的表达水平。SKBR3-PR细胞铺6孔板培养至50%~60%进行相应的实验处理,48 h后用胰酶消化离心,按照沉淀多少加入适量的蛋白裂解液(PMSF∶RIPA=1∶99),静止后离心保存。配置SDS-聚丙烯酰胺凝胶,按照每孔25 μg的蛋白上样量上样,70 V电压跑胶,当跑到浓缩胶与分离胶分界时,更换100 V电压,按照200 mA 1.5 h进行转膜,封闭液封闭2 h后,敷相应一抗过夜,第2天敷二抗,配置显影液显影检测蛋白的表达水平。
-
采用方差分析和t检验。
1.1. 细胞及试剂
1.2. 细胞培养
1.3. LncRNA干扰片段与miRNA-inhibitor转染
1.4. qRT-PCR检测
1.5. 细胞划痕实验
1.6. 细胞凋亡实验
1.7. Western blotting实验
1.8. 统计学方法
-
与亲本细胞株比较,在乳腺癌耐药细胞株中,LncRNA SNHG15表达水平上调,但差异无统计学意义(P>0.05);miRNA-451a表达水平下降,CXCR4表达水平上升, 差异均有统计学意义(P<0.05和P<0.01)(见表 2)。在乳腺癌耐药细胞中转染并敲低SNHG15后miRNA-451a表达水平上调,且差异有统计学意义(P<0.05)(见表 3)。在乳腺癌耐药细胞中转染并抑制miRNA-451a时CXCR4表达水平上调, 且差异有统计学意义(P<0.01)(见表 4)。而当SI-LncRNA与miRNA-451a-inhibitor联合使用时CXCR4的表达量位于两者之间(见表 5)。说明在耐药细胞中SNHG15的表达量与miRNA-451a成负相关,与CXCR4成正相关。
分组 n SNHG15 miRNA-451a CXCR4 SKBR3组 3 1 1 1 SKBR3-PR组 3 2.040±0.285 0.183±0.182 1.550±0.458 t — 6.32 7.78 2.08 P — >0.05 < 0.05 < 0.01 分组 n miRNA-451a SKBR3-PR组 3 1 SI-LncRNA组 3 3.41±0.922 t — 4.53 P — < 0.05 分组 n CXCR4 SKBR3-PR组 3 1 miRNA-451a-inhibitor组 3 3.233±0.440 t — 8.79 P — < 0.01 分组 n CXCR4 SKBR-3-PR组 3 1 SI-LncRNA组 3 0.097±0.086** miRNA-451a-inhibior组 3 3.26±0.441**## SI-LncRNA+miRNA-451a-inhibitor组 3 1.53±0.127**##△△ F — 94.97 P — < 0.01 MS组内 — 0.447 q检验:与SKBR-3-PR组比较**P<0.01与SI-LncRNA组比较##P<0.01;与miRNA-451a-inhibitor组比较△△P<0.01 -
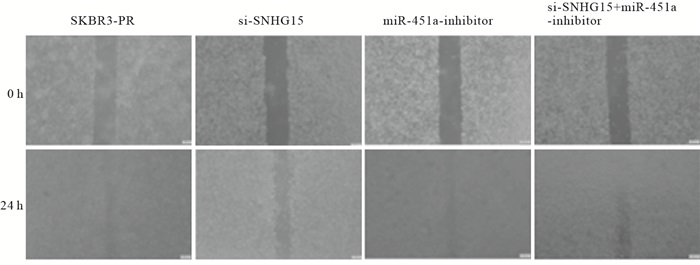
在乳腺癌SKBR3-PR细胞中,转染SI-LncRNA 24 h后,与正常对照组相比较,乳腺癌细胞的伤口愈合能力明显下降,迁移率降低(P<0.01);而转染miRNA-451a-inhibitor后,乳腺癌细胞的伤口较正常对照组变小,迁移能力上升(P<0.01);当SI-LncRNA+miRNA-451a-inhibitor联合使用时,细胞的迁移能力在二者之间(P<0.01)(见图 1、表 6)。
分组 n 迁移率/% SKBR3-PR组 3 56.67±1.12 SI-LncRNA组 3 4.40±1.77** miRNA-451a-inhibitor组 3 57.23±6.09**## SI-LncRNA+miRNA-451a-inhibitor组 3 23.56±2.49**##△△ F — 170.33 P — < 0.01 MS组内 — 11.919 q检验:与SKBR3-PR组比较**P<0.01;与SI-LncRNA组比较##P<0.01, 与miRNA-451a-inhibitor组比较△△P<0.01 -
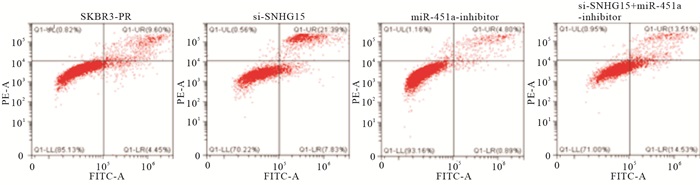
与SKBR3-PR对照组相比,转染SI-LncRNA组晚期细胞凋亡率上升(P<0.01)。而转染miRNA-451a-inhibitor组细晚期胞凋亡率下降(P<0.01),而当SI-LncRNA与miRNA-451a-inhibitor联合使用时,乳腺癌细胞的凋亡率则在二者之间,从而说明二者对SKBR3-PR的凋亡作用呈现相反的作用(P<0.01)(见图 2、表 7)。
分组 n 晚期凋亡率/% SKBR3-PR组 3 9.07±0.55 SI-LncRNA组 3 20.97±1.44** miRNA-451a-inhibitor组 3 5.36±0.58**## SI-LncRNA+miRNA-451a-inhibitor组 3 12.35±1.12**##△△ F — 134.35 P — < 0.01 MS组内 — 0.992 q检验:与SKBR3-PR组比较**P<0.01;与SI-LncRNA组比较##P<0.01, 与miRNA-451a-inhibitor组比较△△P<0.01 -
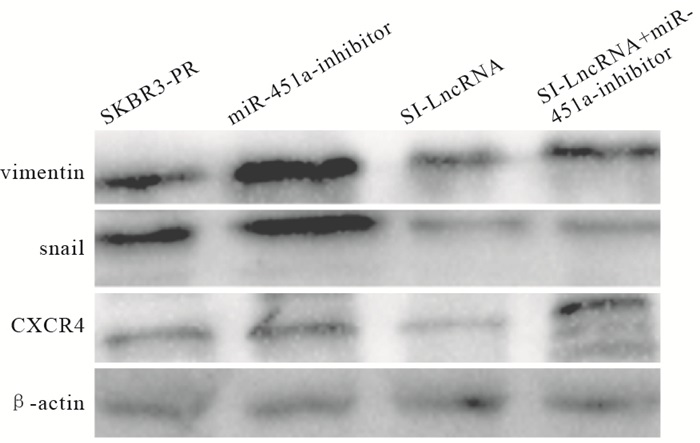
与SKBR3-PR对照组相比,转染SI-LncRNA组,靶基因CXCR4表达量下降(P<0.01),而转染miRNA-451a-inhibitor组表达量上升(P<0.01),EMT相关蛋白组Snail在转染SI-LncRNA时表达量相较对照组有明显下降(P<0.01),转染miRNA-451a-inhibitor组时表达量相较对照组上调(P<0.01), Vimentin指标变化无统计学意义(P>0.05)。当SI-LncRNA与miRNA-451a-inhibitor联合使用时,Snail表达量位于两者之间(P<0.01)(见图 3、表 8)。
分组 n Vimentin Snail CXCR4 SKBR3-PR组 3 1.369±0.039 1.438±0.004 1.071±0.384 SI-LncRNA组 3 1.049±0.123 0.398±0.011** 0.241±0.003** miRNA-451a-inhibitor组 3 1.663±0.986 1.595±0.007**## 1.156±0.006**## SI-LncRNA+miRNA-451a-inhibitor组 3 1.551±0.004 0.387±0.006**##△△ 0.727±0.006**##△△ F — 0.87 22 986.68 14.04 P — >0.05 < 0.01 < 0.01 MS组内 — 0.247 0.000 0.037 q检验:与SKBR3-PR组比较**P<0.01;与SI-LncRNA组比较##P<0.01;与miRNA-451a-inhibitor组比较△△P<0.01
2.1. 耐药细胞中SNHG15、miRNA-451a和CXCR4的表达
2.2. 细胞划痕实验
2.3. 细胞凋亡实验
2.4. Western blotting检测靶基因CXCR4以及EMT相关蛋白表达情况
-
乳腺癌作为严重危害女性生命健康的恶性疾病之一,死亡率占女性第二位[4],目前针对乳腺癌通常使用以放疗化疗为主的手段[5-8],但是由于肿瘤的复发、转移与耐药性的产生,往往导致其治疗失败,近年来更加倾向于以生物治疗为主,寻找新的作用靶点。
LncRNA是一类长度约200个核苷酸的RNA分子,根据互补碱基配对的原理,具有多种二级结构,可与多种蛋白质和核酸特异性结, 近年来越来越多的研究发现,LncRNA与乳腺癌的发生有十分重要的关系,SUN等[9]研究发现,lncRNA H19高表达可促进乳腺癌的生长。DONG等[10-11]使用芯片技术检测曲妥珠单抗耐药及敏感细胞株中LncRNA的表达,发现异常表达的SNHG14和AGAP2-AS1,并证实SNHG14上调可促进乳腺癌细胞增殖和对曲妥珠单抗产生耐药。GÓMEZ-MALDONADO等[12]研究指出,LncRNA可以作为肿瘤抑制因子来调节乳腺癌的浸润、侵袭、转移过程。WANG等[13]研究表明三阴性乳腺癌的LncRNA表达谱中,LncRNA SNHG12表达量显著上调,其表达水平与肿瘤直径与淋巴结转移显著相关。miRNA作为一种微小RNA, 被研究证明可以在乳腺癌中起癌基因或抑癌基因的作用,YE等[14]对MCF-7细胞系中miRNAs的表达进行了分析发现,MCF-7耐药细胞系中miR-27a的表达下降,表明miR-27a的低表达与乳腺癌细胞获得性耐药有密切关系。另有研究[15]表明, miR-125b可以调控HK2增强乳腺癌细胞的敏感性。本研究旨在揭示LncRNA SNHG15对miRNA-451a的调控作用以及引起乳腺癌细胞生物学活性的变化。因此,本实验从三者之间的相互关系作为研究的切入点,首先通过验证发现SNHG15、miRNA-451a、CXCR4三者之间表达量的关系以及相互作用的趋势,其次通过敲低SNHG15以及抑制miRNA-451a的表达,通过划痕实验验证乳腺癌耐药细胞的迁移能力,我们研究发现当敲低了SNHG15后乳腺癌耐药细胞的迁移能力相比于正常组减弱,而抑制miRNA-451a后其迁移能力增强。而通过检测其在乳腺癌细胞的生物学活性可以发现,SNHG15在乳腺癌细胞中起到癌基因的作用,促进了乳腺癌细胞的迁移,并抑制其凋亡,相反miRNA-451a则起到抑癌基因的作用,趋化因子CXCR4在乳腺癌细胞中的表达量与LncRNA SNHG15呈现一致性,而与miRNA-451a呈相反的趋势,而miRNA-451a又与CXCR4抑制剂具有相同的作用。通过Western blotting实验发现:当敲低SNHG15与抑制miRNA-451a联合作用时,EMT指标Snail表达量具有统计学意义。
综上所述,LncRNA SNHG15在乳腺癌细胞的迁移和凋亡能力中发挥了重要的作用。LncRNA SNHG15可能通过调控miRNA-451a表达水平,从而在一定程度上潜在的调控上调CXCR4的表达水平,我们猜测LncRNA SNHG15与miRNA-451a之间存在某种竞争性关系,而二者皆可调控CXCR4的表达。其中具体的作用机制仍未可知, 但可以明确CXCR4的变化在一定程度上引起了乳腺癌细胞的EMT。这一发现可以作为今后临床对于治疗乳腺癌的新型切入点与相关的治疗靶点,为乳腺癌的治愈提供了一定的可能性。








 DownLoad:
DownLoad: