-
慢性肾脏病(chronic kidney disease,CKD)已经成为一种常见病,在全球影响8%~16%的成年人健康[1]。CKD病人进入终末期肾病(ESRD)者大部分选择血液透析(hemodialysis,HD)治疗,自体动静脉内瘘(arteriovenous fistulas, AVF)是HD病人长期透析的最理想的血管通路,而内瘘血管钙化是其失功主要原因之一。血管钙化主要指血管在多种因素作用下引起血管僵硬度增加,血管的弹性降低,可引起血栓形成,斑块破裂等后果[2]。内瘘血管钙化极易造成内瘘血管血栓栓塞、透析时血流动力学异常等各种危险因素引起HD病人无法透析威胁生命。探讨内瘘血管钙化相关影响因素变得尤为重要,其中有研究[3]指出,骨形态发生蛋白-2(BMP-2)/Smad信号通路参与成纤维细胞生长因子21(FGF-21)对血管钙化的抑制作用。由于内瘘血管的特殊性,关于FGF-21及BMP-2在内瘘血管钙化作用研究鲜见报道。本研究旨在探讨FGF-21、BMP-2在维持性血液透析病人自体AVF血管钙化中的作用及其与内瘘血管钙化的相关性。
HTML
-
选择2020年8月至2021年6月收治于我科行内瘘修复术及CKD 4~5期初次内瘘病人各20例为观察组。纳入标准:(1)年龄>18岁;(2)病情稳定;(3)透析时间>3个月(仅限于行内瘘修复病人或非内瘘透析行初次内瘘病人;(4)AVF以头静脉-桡动脉端侧吻合;(5)在我院规律透析病人:每2周透析5次(即每周2次或3次交替)。排除标准:(1)合并乙型肝炎或丙型肝炎、结核等传染性疾病病人;(2)严重疾病的活动期及恶性肿瘤病人;(3)原发性甲状旁腺疾病及严重的周围血管病变等影响血管钙化结果测定的疾病;(4)腹膜透析病人。观察组共40例,其中男22例,女18例;年龄(55.60±10.48)岁,透析龄(39.98±26.17)个月;原发病:慢性肾小球肾炎18例,糖尿病肾病12例,高血压肾损害5例,痛风性肾病1例,多囊肾1例,系统性红斑狼疮性肾炎1例,不明病因2例。择同期我院健康体检中心的健康人群20名作为对照组,其中男10名,女10名。所有研究对象均签署知情同意书且本研究符合我院伦理学标准。
-
所有研究对象采集空腹上肢静脉血, 采用本院自动生化分析仪检测血钙(Ca)、血磷(P)、空腹血糖(GLU)、总胆固醇(TC)、血清肌酐(SCr)、碱性磷酸酶(ALP)、血清尿素氮(BUN)、甲状旁腺素(PTH)、25-羟-维生素D(25-羟-VD)等水平。
-
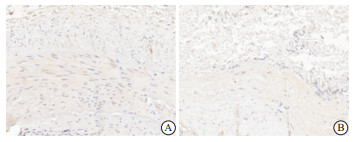
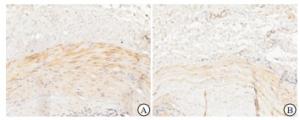
(1) 酶联免疫吸附法(ELISA)检测:采集清晨空腹静脉血,2 h内离心取上层血清并迅速放置于-80 ℃冰箱保存,在室温环境下复融后采用ELISA测定450 nm波长下的FGF-21、BMP-2吸光度值(OD值),通过绘制标准曲线测定血清FGF-21及BMP-2浓度,操作步骤均按试剂盒说明书操作,ELISA试剂盒由合肥安启生物科技有限公司提供。(2)免疫组织化学(免疫组化)染色:按照免疫组化试剂盒操作步骤进行,内瘘血管组织石蜡切片常规脱蜡脱水后,通过热修复抗原后再用5%牛血清白蛋白封闭20 min,分别滴加稀释的BMP-2抗体、FGF-21抗体于4 ℃过夜,次日滴加二抗37 ℃孵育20 min,二氨基联苯胺显色,苏木精复染,透明,中性树脂封片在显微镜下观察。其中每项检测均以磷酸盐缓冲液代替一抗作为阴性对照。通过Image-J分析软件分析在光镜下呈黄色颗粒性沉积区域及程度,无细胞着色为0分,弱阳性为1分,中等阳性为2分,强阳性为3分;结合FGF-21、BMP-2染色阳性细胞密度进行评分,阳性细胞数≤25%计1分,26%~50%计2分,51%~75%计3分,76%~100%计4分。以S表示两项乘积进而计算FGF-21及BMP-2因子免疫组织化学积分,≤7分为阴性组,>7分为阳性组,进而观察实验因子的表达情况。检测试剂所用的一抗、二抗体均由合肥安启生物科技有限公司提供。
-
取观察组病人内瘘血管组织于10%中性甲醛固定、石蜡包埋、切片。采用钙盐特异性染色法(茜素红S法)对其进行钙化染色, 操作步骤均按试剂盒说明书操作,表现为橙红色即为钙化染色阳性。对每例切片染色8片,根据钙化积分取平均值的标准: < 1为无钙化,1~2.5为轻中度钙化,>2.5~4为重度钙化,对切片进行评分,根据钙化积分将观察组病人分为无钙化组、钙化组(轻、中度钙化组和重度钙化组)。
-
由本院专业技术人员采用我院动脉硬化全自动检测仪BP-203RPE-Ⅲ(PWV/ABI型)检测baPWV值,取左右两侧baPWV的高值进行统计分析。
-
采用t检验、单因素方差分析和q检验、χ2检验及Spearman相关分析。
1.1. 一般资料
1.2. 方法
1.2.1. 生化指标的检测
1.2.2. FGF-21、BMP-2的检测
1.2.3. 内瘘血管钙化的评估
1.2.4. 肱踝脉搏波传导速度(baPWV)评估全身血管硬化情况
1.3. 统计学方法
-
初次内瘘组、内瘘修复组和对照组3组间比较,除性别、GLU水平差异无统计学意义外(P>0.05), 其他临床相关指标差异均有统计学意义(P < 0.01)(见表 1)。
指标 对照组
(n=20)初次内瘘组
(n=20)内瘘修复组
(n=20)F P MS组内 男[n;百分率(%)] 10(50.00) 9(45.00) 13(65.00) 1.74* >0.05 — 年龄/岁 45.20±12.05 56.80±10.89△△ 54.40±10.18△△ 6.12 < 0.01 122.476 P/(mmol/L) 1.30±0.09 1.64±0.03△△ 2.13±0.67△△## 35.78 < 0.01 0.173 Ca/(mmol/L) 2.29±0.10 2.08±0.15△△ 2.15±0.22# 15.56 < 0.01 0.028 GLU/(mmol/L) 5.45±2.05 5.85±3.41 6.00±2.40 0.41 >0.05 7.220 TC/(mmol/L) 4.88±0.91 3.62±1.10△△ 3.94±0.96△△ 16.83 < 0.01 0.985 BUN/(mmol/L) 5.16±1.07 25.94±9.69△△ 22.71±6.14△△ 39.64 < 0.01 44.226 SCr/(μmol/L) 61.85±8.41 571.95±212.39△△ 774.85±227.19△△# 83.68 < 0.01 32 254.597 ALP/(U/L) 57.10±17.02 80.60±31.96△ 98.00±40.04△△ 16.94 < 0.01 971.589 PTH/(ng/L) 47.61±4.05 164.24±95.76△△ 333.94±235.46△△# 33.19 < 0.01 21 544.362 25-羟-VD/(ng/mL) 25.18±2.14 18.02±5.17△△ 15.41±5.93△△ 27.66 < 0.01 22.154 FGF-21/(pg/mL) 50.09±9.46 180.43±110.87△△ 292.42±217.55△△ 37.54 < 0.01 19 903.652 BMP-2/(pg/mL) 6.49±1.24 41.22±25.31△△ 43.17±20.05△△ 38.48 < 0.01 348.072 baPWV/(cm/s) 1 273.70±174.43 1 699.50±237.37△△ 1 794.00±124.48△△ 31.80 < 0.01 34 087.319 *示χ2值; q检验:与对照组比较△P < 0.05,△△P < 0.01;与初次内瘘组比较#P < 0.05,## P < 0.01 -
观察组40例中内瘘血管钙化病人共24例,发生率达60.00%。内瘘血管钙化病人血FGF-21、BMP-2、P、PTH、SCr水平及baPWV值均明显高于内瘘血管无钙化病人(P < 0.05~P < 0.01),Ca水平明显低于无钙化组(P < 0.01), 其他临床指标相比较差异均无统计学意义(见表 2)。
指标 无钙化组
(n=16)轻、中度组
(n=15)重度组
(n=9)F P MS组内 男[n;百分率(%)] 10(62.50) 7(46.70) 5(55.50) 0.79* >0.05 — 年龄/岁 52.3±12.83 57.53±8.17 58.22±8.54 1.35 >0.05 107.803 P/(mmol/L) 1.53±0.17 1.85±0.47△ 2.55±0.58△△## 21.95 < 0.01 0.165 Ca/(mmol/L) 2.16±0.14 2.15±0.20 1.94±0.20△△## 17.43 < 0.05 0.031 GLU/(mmol/L) 5.63±2.07 6.40±3.85 5.65±2.61 0.31 >0.05 8.824 TC/(mmol/L) 3.76±1.08 3.71±0.92 3.94±1.21 0.44 >0.05 1.113 BUN/(mmol/L) 23.99±9.20 23.26±8.44 26.69±5.80 0.51 >0.05 68.492 SCr/(μmol/L) 578.81±243.56 625.87±152.61 926.11±184.39△△## 9.46 < 0.01 40 211.380 ALP/(U/L) 82.69±21.22 87.93±39.72 103.33±51.92 0.67 >0.05 1 362.280 PTH/(ng/L) 180.93±95.87 194.47±134.07 461.28±274.29△△## 4.24 < 0.05 26 794.571 25-羟-VD/(ng/mL) 17.20±4.69 16.71±5.26 15.58±8.03 2.20 >0.05 33.309 FGF-21/(pg/mL) 105.15±41.33 210.93±69.77△△ 512.28±155.75△△## 29.87 < 0.01 7 779.578 BMP-2/(pg/mL) 22.58±6.72 45.22±12.11△△ 71.99±18.82△△## 36.31 < 0.01 150.427 baPWV/(cm/s) 1 628.80±207.50 1 822.80±145.12△△ 1 829.00±133.5△△ 6.33 < 0.05 29 282.587 *示χ2值; q检验:与无钙化组比较△P < 0.05,△△P < 0.01;与轻、中度组比较## P < 0.01 -
观察组与对照组baPWV存在明显差异,初次内瘘组baPWV为(1 699.50±237.37)cm/s, 内瘘修复组为(1 794.00±124.48)cm/s, 对照组为(1 273.70±174.43)cm/s, 差异有统计学意义(P < 0.01);内瘘血管钙化病人baPWV大于非钙化血管病人(P < 0.01)(见表 1、2)。
-
Spearman相关分析显示,观察组病人内瘘血管钙化与血清FGF-21、BMP-2、P、SCr、PTH、baPWV均呈正相关(rs=0.850、0.769、0.718、0.433、0.441、0.457,P < 0.01),与Ca、GLU、TC、BUN、ALP、25-羟-VD、性别、年龄指标无相关性(rs=-0.398、-0.113、0.014、0.244、0.006、-0.278、0.084、0.133,P>0.05)。
-
通过观察所有AVF血管钙化的标本,FGF-21及BMP-2在钙化血管壁上均有所表达,大都表现于血管中膜,重度钙化血管表达量明显大于轻、中度钙化血管(见图 1~2)。重度钙化组FGF-21、BMP-2阳性表达高于轻、中度钙化组(P < 0.05)(见表 3)。
分组 n S/FGF-21 S/BMP-2 重度钙化组 9 10.67±0.67 9.67±0.60 轻、中度钙化组 15 8.53±0.27 8.26±0.11 t — 2.96 2.29 P — < 0.05 < 0.05
2.1. 所有研究对象临床相关指标情况
2.2. 观察组不同亚组间临床相关指标的比较
2.3. baPWV评估全身血管硬化情况分析
2.4. 内瘘血管钙化与临床相关指标的相关性分析
2.5. 免疫组化分析结果
-
自体AVF血管通路是CKD病人HD的生命线,是通过手术使得上肢表浅动脉与静脉之间相互吻合,促使原始静脉壁变厚及管腔扩展达到“静脉动脉化”后进而成熟使用。本研究选择的组织标本均来源病人的“动脉化”的头静脉及初瘘的头静脉组织。目前关于CKD病人内瘘血管钙化原因大都归因于病人钙磷代谢紊乱、脂质浸润、氧化应激和慢性炎症等原因的不断刺激,不可忽视的是CKD病人血管钙化的病理生理基础更考虑是类似于骨形成的主动调节过程[4],主要表现为血管平滑肌细胞发生成骨样变化。本研究中内瘘血管钙化病人不仅钙磷代谢失调严重,血清FGF-21及BMP-2水平均高于正常人及内瘘血管无钙化病人。
FGF-21是FGF家族的成员, 其基因位于人类19号染色体且有4个外显子编码209个氨基酸的肽(前FGF-21),于2000年首次被鉴定,与鼠FGF-21有75%的同源性[5]。成熟的FGF-21是切割28个氨基酸的N端信号肽后形成一种181个氨基酸的蛋白质,相对分子质量约为20 kDa[6]。关于尿中FGF21水平的数据很少[7],根据推断,肾清除率仍被认为是其主要排泄途径,对于CKD病人,循环中的FGF-21水平与SCr呈正相关,与肾小球滤过率呈负相关,且从早期肾病发展到后期ESRD状态,FGF-21水平不断升高[8]。本研究中也证实随着病人疾病进展,FGF-21水平不断增高。FGF-21作用的实施需要借助FGF受体和辅助因子β-Klotho(β-Klotho是一种单通道跨膜蛋白,是FGF-21信号传递的专属辅因子)[9]。FGF-21通过减少矿物质沉积[10]和内质网应激介导的凋亡[11],降低成骨细胞基因的表达和抑制血管平滑肌细胞的成骨转化[12]来抑制血管钙化及其进展。FGF-21通过OPG/RANKL系统抑制血管钙化[13-14], 且能够显著下调Runx2和ALP的表达,这两种蛋白是成骨细胞分化的标志物和积极调节因子[15-16]。随着血管钙化程度的加重,血清FGF-21不断升高进而抑制血管钙化的进展。本研究中亦观察到内瘘血管钙化组血清中FGF-21表达高于内瘘无钙化组,且FGF-21在钙化程度越高的血管壁上的免疫组织表达也越强,与上述关于FGF-21在慢性肾脏病中的研究结果一致。
BMP属于转化生长因子β超家族成员[17]且是一种多功能的生长因子,其中BMP-2是成骨细胞分化和骨形成所需的有效成骨蛋白。BMP-2主要通过依赖于Ⅰ型和Ⅱ型受体以及细胞内的信号分子Smad发挥生物学效应,BMP-2/Smad信号通路在成骨细胞分化和骨形成中起关键作用[18]。在该途径中,BMP-2配体结合并激活跨膜受体Ⅰ(BMPR-ⅠA) 和Ⅱ(BMPR-Ⅱ)[19],然后激活的BMPR-ⅠA通过Smad1/5/8(p-Smad1/5/8)[20]的募集和磷酸化来传递信号进而转移至细胞核内调节Runx2和ALP的表达来引起血管的钙化。由于动静脉内瘘血管的特殊性,可通过BMP-2的血清值及在血管壁上的表达情况反映病人血管的钙化程度。在本次研究中可观察到内瘘血管钙化病人血清中BMP-2水平明显高于内瘘血管无钙化病人且在不同钙化水平血管壁上的表达存在一定差异。
综上所述,本研究可证实FGF-21、BMP-2水平升高与自体AVF血管钙化相关,可将其作为预测内瘘病人血管钙化的参考指标。但本研究由于样本量较小及研究时间与条件有限,存在一定的限制性,后期仍需要扩大样本量做更深入层次研究。








 DownLoad:
DownLoad: